Mitochondria-Translocated PGK1 Functions as a Protein Kinase to Coordinate Glycolysis and the TCA Cycle in Tumorigenesis
- PMID: 26942675
- PMCID: PMC4888784
- DOI: 10.1016/j.molcel.2016.02.009
Mitochondria-Translocated PGK1 Functions as a Protein Kinase to Coordinate Glycolysis and the TCA Cycle in Tumorigenesis
Abstract
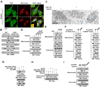
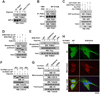
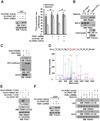
It is unclear how the Warburg effect that exemplifies enhanced glycolysis in the cytosol is coordinated with suppressed mitochondrial pyruvate metabolism. We demonstrate here that hypoxia, EGFR activation, and expression of K-Ras G12V and B-Raf V600E induce mitochondrial translocation of phosphoglycerate kinase 1 (PGK1); this is mediated by ERK-dependent PGK1 S203 phosphorylation and subsequent PIN1-mediated cis-trans isomerization. Mitochondrial PGK1 acts as a protein kinase to phosphorylate pyruvate dehydrogenase kinase 1 (PDHK1) at T338, which activates PDHK1 to phosphorylate and inhibit the pyruvate dehydrogenase (PDH) complex. This reduces mitochondrial pyruvate utilization, suppresses reactive oxygen species production, increases lactate production, and promotes brain tumorigenesis. Furthermore, PGK1 S203 and PDHK1 T338 phosphorylation levels correlate with PDH S293 inactivating phosphorylation levels and poor prognosis in glioblastoma patients. This work highlights that PGK1 acts as a protein kinase in coordinating glycolysis and the tricarboxylic acid (TCA) cycle, which is instrumental in cancer metabolism and tumorigenesis.
Keywords: B-Raf; EGFR; K-Ras; PDH; PDHK1; PGK1; glycolysis; hypoxia; mitochondria; phosphorylation; tumorigenesis.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
O-GlcNAcylation of PGK1 coordinates glycolysis and TCA cycle to promote tumor growth.Nat Commun. 2020 Jan 7;11(1):36. doi: 10.1038/s41467-019-13601-8. Nat Commun. 2020. PMID: 31911580 Free PMC article.
-
Hypoxia-induced downregulation of PGK1 crotonylation promotes tumorigenesis by coordinating glycolysis and the TCA cycle.Nat Commun. 2024 Aug 12;15(1):6915. doi: 10.1038/s41467-024-51232-w. Nat Commun. 2024. PMID: 39134530 Free PMC article.
-
PTEN Suppresses Glycolysis by Dephosphorylating and Inhibiting Autophosphorylated PGK1.Mol Cell. 2019 Nov 7;76(3):516-527.e7. doi: 10.1016/j.molcel.2019.08.006. Epub 2019 Sep 3. Mol Cell. 2019. PMID: 31492635
-
Resistance to anoikis in transcoelomic shedding: the role of glycolytic enzymes.Pleura Peritoneum. 2019 Mar 12;4(1):20190003. doi: 10.1515/pp-2019-0003. eCollection 2019 Mar 1. Pleura Peritoneum. 2019. PMID: 31198853 Free PMC article. Review.
-
Microenvironmental control of glucose metabolism in tumors by regulation of pyruvate dehydrogenase.Int J Cancer. 2019 Feb 15;144(4):674-686. doi: 10.1002/ijc.31812. Epub 2018 Oct 4. Int J Cancer. 2019. PMID: 30121950 Free PMC article. Review.
Cited by
-
Metabolic Signature of Warburg Effect in Cancer: An Effective and Obligatory Interplay between Nutrient Transporters and Catabolic/Anabolic Pathways to Promote Tumor Growth.Cancers (Basel). 2024 Jan 24;16(3):504. doi: 10.3390/cancers16030504. Cancers (Basel). 2024. PMID: 38339256 Free PMC article. Review.
-
Comparison of Mitochondrial and Antineoplastic Effects of Amiodarone and Desethylamiodarone in MDA-MB-231 Cancer Line.Int J Mol Sci. 2024 Sep 10;25(18):9781. doi: 10.3390/ijms25189781. Int J Mol Sci. 2024. PMID: 39337269 Free PMC article.
-
Role of Mitochondria-Cytoskeleton Interactions in the Regulation of Mitochondrial Structure and Function in Cancer Stem Cells.Cells. 2020 Jul 14;9(7):1691. doi: 10.3390/cells9071691. Cells. 2020. PMID: 32674438 Free PMC article. Review.
-
Hypoxia and Selective Autophagy in Cancer Development and Therapy.Front Cell Dev Biol. 2018 Sep 10;6:104. doi: 10.3389/fcell.2018.00104. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 30250843 Free PMC article. Review.
-
The improved energy metabolism and blood oxygen-carrying capacity for pufferfish, Takifugu fasciatus, against acute hypoxia under the regulation of oxygen sensors.Fish Physiol Biochem. 2019 Feb;45(1):323-340. doi: 10.1007/s10695-018-0565-2. Epub 2018 Sep 17. Fish Physiol Biochem. 2019. PMID: 30225749
References
-
- Ahmad SS, Glatzle J, Bajaeifer K, Buhler S, Lehmann T, Konigsrainer I, Vollmer JP, Sipos B, Ahmad SS, Northoff H, et al. Phosphoglycerate kinase 1 as a promoter of metastasis in colon cancer. International journal of oncology. 2013 - PubMed
-
- Ai J, Huang H, Lv X, Tang Z, Chen M, Chen T, Duan W, Sun H, Li Q, Tan R, et al. FLNA and PGK1 are two potential markers for progression in hepatocellular carcinoma. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2011;27:207–216. - PubMed
-
- Bernstein BE, Hol WG. Crystal structures of substrates and products bound to the phosphoglycerate kinase active site reveal the catalytic mechanism. Biochemistry. 1998;37:4429–4436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA082683/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- 1R0 CA169603/CA/NCI NIH HHS/United States
- CA82683/CA/NCI NIH HHS/United States
- 2P50CA127001/CA/NCI NIH HHS/United States
- CA16672/CA/NCI NIH HHS/United States
- 2R01 CA109035/CA/NCI NIH HHS/United States
- R01 CA169603/CA/NCI NIH HHS/United States
- R01 CA109035/CA/NCI NIH HHS/United States
- 1R01NS089754/NS/NINDS NIH HHS/United States
- R01 NS089754/NS/NINDS NIH HHS/United States
- P50 CA127001/CA/NCI NIH HHS/United States
- P30 CA014195/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

