Opposing effects of Sca-1(+) cell-based systemic FGF2 gene transfer strategy on lumbar versus caudal vertebrae in the mouse
- PMID: 26934099
- PMCID: PMC4891288
- DOI: 10.1038/gt.2016.21
Opposing effects of Sca-1(+) cell-based systemic FGF2 gene transfer strategy on lumbar versus caudal vertebrae in the mouse
Abstract
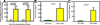
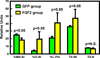
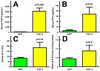
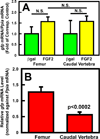
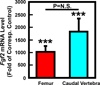
Our previous work showed that a Sca-1(+) cell-based FGF2 therapy was capable of promoting robust increases in trabecular bone formation and connectivity on the endosteum of long bones. Past work reported that administration of FGF2 protein promoted bone formation in red marrow but not in yellow marrow. The issue as to whether the Sca-1(+) cell-based FGF2 therapy is effective in yellow marrow is highly relevant to its clinical potential for osteoporosis, as most red marrows in a person of an advanced age are converted to yellow marrows. Accordingly, this study sought to compare the osteogenic effects of this stem cell-based FGF2 therapy on red marrow-filled lumbar vertebrae with those on yellow marrow-filled caudal vertebrae of young adult W(41)/W(41) mice. The Sca-1(+) cell-based FGF2 therapy drastically increased trabecular bone formation in lumbar vertebrae, but the therapy not only did not promote bone formation but instead caused substantial loss of trabecular bone in caudal vertebrae. The lack of an osteogenic response was not due to insufficient engraftment of FGF2-expressing Sca-1(+) cells or inadequate FGF2 expression in caudal vertebrae. Previous studies have demonstrated that recipient mice of this stem cell-based FGF2 therapy developed secondary hyperparathyroidism and increased bone resorption. Thus, the loss of bone mass in caudal vertebrae might in part be due to an increase in resorption without a corresponding increase in bone formation. In conclusion, the Sca-1(+) cell-based FGF2 therapy is osteogenic in red marrow but not in yellow marrow.
Conflict of interest statement
Figures


Similar articles
-
Stem cell antigen-1 positive cell-based systemic human growth hormone gene transfer strategy increases endosteal bone resorption and bone loss in mice.J Gene Med. 2011 Feb;13(2):77-88. doi: 10.1002/jgm.1542. J Gene Med. 2011. PMID: 21322098
-
Stem cell antigen-1+ cell-based bone morphogenetic protein-4 gene transfer strategy in mice failed to promote endosteal bone formation.J Gene Med. 2009 Oct;11(10):877-88. doi: 10.1002/jgm.1369. J Gene Med. 2009. PMID: 19629966
-
Erythroid promoter confines FGF2 expression to the marrow after hematopoietic stem cell gene therapy and leads to enhanced endosteal bone formation.PLoS One. 2012;7(5):e37569. doi: 10.1371/journal.pone.0037569. Epub 2012 May 18. PLoS One. 2012. PMID: 22629419 Free PMC article.
-
Young bone marrow Sca-1 cells protect aged retina from ischaemia-reperfusion injury through activation of FGF2.J Cell Mol Med. 2018 Dec;22(12):6176-6189. doi: 10.1111/jcmm.13905. Epub 2018 Sep 25. J Cell Mol Med. 2018. PMID: 30255622 Free PMC article.
-
Sca-1(+) hematopoietic cell-based gene therapy with a modified FGF-2 increased endosteal/trabecular bone formation in mice.Mol Ther. 2007 Oct;15(10):1881-9. doi: 10.1038/sj.mt.6300258. Epub 2007 Jul 17. Mol Ther. 2007. PMID: 17637718
Cited by
-
Endogenous FGF-2 levels impact FGF-2/BMP-2 growth factor delivery dosing in aged murine calvarial bone defects.J Biomed Mater Res A. 2021 Dec;109(12):2545-2555. doi: 10.1002/jbm.a.37249. Epub 2021 Jun 26. J Biomed Mater Res A. 2021. PMID: 34173706 Free PMC article.
-
FGF/FGFR signaling in health and disease.Signal Transduct Target Ther. 2020 Sep 2;5(1):181. doi: 10.1038/s41392-020-00222-7. Signal Transduct Target Ther. 2020. PMID: 32879300 Free PMC article. Review.
References
-
- Kalpakcioglu BB, Morshed S, Engelke K, Genant HK. Advanced imaging of bone macrostructure and microstructure in bone fragility and fracture repair. J Bone Joint Surg Am. 2008;90(Suppl 1):68–78. - PubMed
-
- Iwamoto J, Takeda T, Sato Y. Efficacy and safety of alendronate and risedronate for postmenopausal osteoporosis. Curr Med Res Opin. 2006;22:919–928. - PubMed
-
- Spangrude GJ, Brooks DM, Tumas DB. Long-term repopulation of irradiated mice with limiting numbers of purified hematopoietic stem cells: in vivo expansion of stem cell phenotype but not function. Blood. 1995;85:1006–1016. - PubMed
-
- Hall SL, Lau KH, Chen ST, Felt JC, Gridley DS, Yee JK, et al. An improved mouse Sca-1+ cell-based bone marrow transplantation model for use in gene- and cell-based therapeutic studies. Acta Haematol. 2007;117:24–33. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

