Cyclic nucleotide-gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in Arabidopsis
- PMID: 26929345
- PMCID: PMC4801260
- DOI: 10.1073/pnas.1524629113
Cyclic nucleotide-gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in Arabidopsis
Abstract
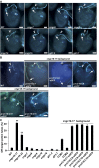
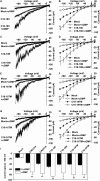
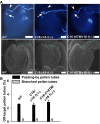
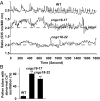
In flowering plants, pollen tubes are guided into ovules by multiple attractants from female gametophytes to release paired sperm cells for double fertilization. It has been well-established that Ca(2+) gradients in the pollen tube tips are essential for pollen tube guidance and that plasma membrane Ca(2+) channels in pollen tube tips are core components that regulate Ca(2+) gradients by mediating and regulating external Ca(2+) influx. Therefore, Ca(2+) channels are the core components for pollen tube guidance. However, there is still no genetic evidence for the identification of the putative Ca(2+) channels essential for pollen tube guidance. Here, we report that the point mutations R491Q or R578K in cyclic nucleotide-gated channel 18 (CNGC18) resulted in abnormal Ca(2+) gradients and strong pollen tube guidance defects by impairing the activation of CNGC18 in Arabidopsis. The pollen tube guidance defects of cngc18-17 (R491Q) and of the transfer DNA (T-DNA) insertion mutant cngc18-1 (+/-) were completely rescued by CNGC18. Furthermore, domain-swapping experiments showed that CNGC18's transmembrane domains are indispensable for pollen tube guidance. Additionally, we found that, among eight Ca(2+) channels (including six CNGCs and two glutamate receptor-like channels), CNGC18 was the only one essential for pollen tube guidance. Thus, CNGC18 is the long-sought essential Ca(2+) channel for pollen tube guidance in Arabidopsis.
Keywords: Arabidopsis; CNGC18; Ca2+ channel; Ca2+ gradient; pollen tube guidance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Cyclic nucleotide-gated channel 18 functions as an essential Ca2+ channel for pollen germination and pollen tube growth in Arabidopsis.Plant Signal Behav. 2017 Nov 2;12(11):e1197999. doi: 10.1080/15592324.2016.1197999. Epub 2016 Jun 20. Plant Signal Behav. 2017. PMID: 27322818 Free PMC article.
-
A calcium-dependent protein kinase interacts with and activates a calcium channel to regulate pollen tube growth.Mol Plant. 2014 Feb;7(2):369-76. doi: 10.1093/mp/sst125. Epub 2013 Oct 11. Mol Plant. 2014. PMID: 24121288
-
A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen.Proc Natl Acad Sci U S A. 2007 Sep 4;104(36):14531-6. doi: 10.1073/pnas.0701781104. Epub 2007 Aug 28. Proc Natl Acad Sci U S A. 2007. PMID: 17726111 Free PMC article.
-
The mechanism and key molecules involved in pollen tube guidance.Annu Rev Plant Biol. 2015;66:393-413. doi: 10.1146/annurev-arplant-043014-115635. Epub 2015 Jan 19. Annu Rev Plant Biol. 2015. PMID: 25621518 Review.
-
Attraction of tip-growing pollen tubes by the female gametophyte.Curr Opin Plant Biol. 2011 Oct;14(5):614-21. doi: 10.1016/j.pbi.2011.07.010. Epub 2011 Aug 19. Curr Opin Plant Biol. 2011. PMID: 21855396 Review.
Cited by
-
Delineating Calcium Signaling Machinery in Plants: Tapping the Potential through Functional Genomics.Curr Genomics. 2021 Dec 30;22(6):404-439. doi: 10.2174/1389202922666211130143328. Curr Genomics. 2021. PMID: 35340362 Free PMC article. Review.
-
A quantitative hypermorphic CNGC allele confers ectopic calcium flux and impairs cellular development.Elife. 2017 Sep 21;6:e25012. doi: 10.7554/eLife.25012. Elife. 2017. PMID: 28933692 Free PMC article.
-
The Long Journey of Pollen Tube in the Pistil.Int J Mol Sci. 2018 Nov 9;19(11):3529. doi: 10.3390/ijms19113529. Int J Mol Sci. 2018. PMID: 30423936 Free PMC article. Review.
-
The Role of Calcium/Calcium-Dependent Protein Kinases Signal Pathway in Pollen Tube Growth.Front Plant Sci. 2021 Mar 9;12:633293. doi: 10.3389/fpls.2021.633293. eCollection 2021. Front Plant Sci. 2021. PMID: 33767718 Free PMC article. Review.
-
From gametes to zygote: Mechanistic advances and emerging possibilities in plant reproduction.Plant Physiol. 2024 Apr 30;195(1):4-35. doi: 10.1093/plphys/kiae125. Plant Physiol. 2024. PMID: 38431529 Free PMC article. Review. No abstract available.
References
-
- Higashiyama T, Takeuchi H. The mechanism and key molecules involved in pollen tube guidance. Annu Rev Plant Biol. 2015;66:393–413. - PubMed
-
- Dresselhaus T, Márton ML. Micropylar pollen tube guidance and burst: Adapted from defense mechanisms? Curr Opin Plant Biol. 2009;12(6):773–780. - PubMed
-
- Dresselhaus T, Franklin-Tong N. Male-female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Mol Plant. 2013;6(4):1018–1036. - PubMed
-
- Wu HM, Wang H, Cheung AY. A pollen tube growth stimulatory glycoprotein is deglycosylated by pollen tubes and displays a glycosylation gradient in the flower. Cell. 1995;82(3):395–403. - PubMed
-
- Palanivelu R, Brass L, Edlund AF, Preuss D. Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell. 2003;114(1):47–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

