Evaluation of phenotypic, functional and molecular characteristics of porcine mesenchymal stromal/stem cells depending on donor age, gender and tissue source
- PMID: 26922917
- PMCID: PMC4937159
- DOI: 10.1292/jvms.15-0596
Evaluation of phenotypic, functional and molecular characteristics of porcine mesenchymal stromal/stem cells depending on donor age, gender and tissue source
Abstract
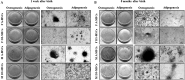
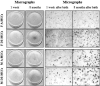
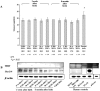
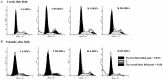
The biological properties of mesenchymal stem cells (MSCs) are influenced by donor age, gender and/or tissue sources. The present study investigated the cellular and molecular properties of porcine mesenchymal stromal/stem cells (MSCs) isolated from different tissues (adipose & dermal skin) and sex at different ages (1 week & 8 months after birth) with similar genetic and environmental backgrounds. MSCs were analyzed for alkaline phosphatase (AP) activity, CD90 and Oct3/4 expression, in vitro differentiation ability, senescence-associated β-galactosidase (SA-β-Gal) activity, telomeric properties, cell cycle status and expression of senescence (IL6, c-myc, TGFβ, p53 and p21)- and apoptosis (Bak and Bcl2)-related proteins. An age-dependent decline in AP activity and adipogenesis was observed in all MSCs, except for male A-MSCs. CD90 expression did not change, but SA-β-Gal activity increased with advancement in age, except in A-MSCs. Telomeric properties were similar in all MSCs, whereas expression levels of Oct3/4 protein declined with the advancement in age. p21 expression was increased with increase in donor age. Male derived cells have shown higher IL6 expression. The expression of p53 was slightly lower in MSCs of dermal tissue than in adipose tissue. Bak was expressed in all MSCs regardless of age, but up regulation of Bcl2 was observed in DS-MSCs derived at 1 week after birth. In conclusion, adipose tissue-derived MSCs from young female individuals were found to be more resistant to senescence under in vitro culture conditions.
Figures

Similar articles
-
Donor-matched functional and molecular characterization of canine mesenchymal stem cells derived from different origins.Cell Transplant. 2013;22(12):2311-21. doi: 10.3727/096368912X657981. Epub 2012 Oct 12. Cell Transplant. 2013. PMID: 23068964
-
Effects of donor age, gender, and in vitro cellular aging on the phenotypic, functional, and molecular characteristics of mouse bone marrow-derived mesenchymal stem cells.Stem Cells Dev. 2011 Sep;20(9):1549-61. doi: 10.1089/scd.2010.0280. Epub 2011 Feb 15. Stem Cells Dev. 2011. PMID: 21204633
-
Iberian pig mesenchymal stem/stromal cells from dermal skin, abdominal and subcutaneous adipose tissues, and peripheral blood: in vitro characterization and migratory properties in inflammation.Stem Cell Res Ther. 2018 Jul 4;9(1):178. doi: 10.1186/s13287-018-0933-y. Stem Cell Res Ther. 2018. PMID: 29973295 Free PMC article.
-
Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue.Int J Mol Med. 2016 Jan;37(1):115-25. doi: 10.3892/ijmm.2015.2413. Epub 2015 Nov 19. Int J Mol Med. 2016. PMID: 26719857 Free PMC article.
-
Mesenchymal stem cells: Identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche.Methods. 2016 Apr 15;99:62-8. doi: 10.1016/j.ymeth.2015.09.016. Epub 2015 Sep 15. Methods. 2016. PMID: 26384580 Review.
Cited by
-
Gender and age-related cell compositional differences in C57BL/6 murine adipose tissue stromal vascular fraction.Adipocyte. 2018;7(3):183-189. doi: 10.1080/21623945.2018.1460009. Epub 2018 Jun 8. Adipocyte. 2018. PMID: 29882687 Free PMC article.
-
Adipose Tissue-Derived Mesenchymal Stem Cells Extend the Lifespan and Enhance Liver Function in Hepatocyte Organoids.Int J Mol Sci. 2023 Oct 21;24(20):15429. doi: 10.3390/ijms242015429. Int J Mol Sci. 2023. PMID: 37895114 Free PMC article.
-
[Research progress of the donor factors and experimental factors affecting adipogenic differentiation of adipose derived stem cells].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017 Nov 15;31(11):1390-1395. doi: 10.7507/1002-1892.201704057. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017. PMID: 29798597 Free PMC article. Chinese.
-
Properties of porcine adipose-derived stem cells and their applications in preclinical models.Adipocyte. 2017 Jul 3;6(3):217-223. doi: 10.1080/21623945.2017.1312040. Epub 2017 Mar 30. Adipocyte. 2017. PMID: 28410000 Free PMC article. Review.
-
Concentrated Secretome of Adipose Stromal Cells Limits Influenza A Virus-Induced Lung Injury in Mice.Cells. 2021 Mar 24;10(4):720. doi: 10.3390/cells10040720. Cells. 2021. PMID: 33804896 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

