β3 Adrenergic Stimulation Restores Nitric Oxide/Redox Balance and Enhances Endothelial Function in Hyperglycemia
- PMID: 26896479
- PMCID: PMC4802476
- DOI: 10.1161/JAHA.115.002824
β3 Adrenergic Stimulation Restores Nitric Oxide/Redox Balance and Enhances Endothelial Function in Hyperglycemia
Abstract
Background: Perturbed balance between NO and O2 (•-). (ie, NO/redox imbalance) is central in the pathobiology of diabetes-induced vascular dysfunction. We examined whether stimulation of β3 adrenergic receptors (β3 ARs), coupled to endothelial nitric oxide synthase (eNOS) activation, would re-establish NO/redox balance, relieve oxidative inhibition of the membrane proteins eNOS and Na(+)-K(+) (NK) pump, and improve vascular function in a new animal model of hyperglycemia.
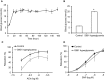
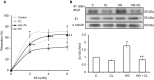
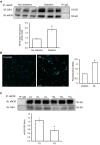
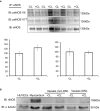
Methods and results: We established hyperglycemia in male White New Zealand rabbits by infusion of S961, a competitive high-affinity peptide inhibitor of the insulin receptor. Hyperglycemia impaired endothelium-dependent vasorelaxation by "uncoupling" of eNOS via glutathionylation (eNOS-GSS) that was dependent on NADPH oxidase activity. Accordingly, NO levels were lower while O2 (•-) levels were higher in hyperglycemic rabbits. Infusion of the β3 AR agonist CL316243 (CL) decreased eNOS-GSS, reduced O2 (•-), restored NO levels, and improved endothelium-dependent relaxation. CL decreased hyperglycemia-induced NADPH oxidase activation as suggested by co-immunoprecipitation experiments, and it increased eNOS co-immunoprecipitation with glutaredoxin-1, which may reflect promotion of eNOS de-glutathionylation by CL. Moreover, CL reversed hyperglycemia-induced glutathionylation of the β1 NK pump subunit that causes NK pump inhibition, and improved K(+)-induced vasorelaxation that reflects enhancement in NK pump activity. Lastly, eNOS-GSS was higher in vessels of diabetic patients and was reduced by CL, suggesting potential significance of the experimental findings in human diabetes.
Conclusions: β3 AR activation restored NO/redox balance and improved endothelial function in hyperglycemia. β3 AR agonists may confer protection against diabetes-induced vascular dysfunction.
Keywords: endothelial dysfunction; endothelial nitric oxide synthase; hyperglycemia; oxidative stress; β3 adrenergic receptors.
© 2016 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures



Similar articles
-
β3-Adrenoceptor activation relieves oxidative inhibition of the cardiac Na+-K+ pump in hyperglycemia induced by insulin receptor blockade.Am J Physiol Cell Physiol. 2015 Sep 1;309(5):C286-95. doi: 10.1152/ajpcell.00071.2015. Epub 2015 Jun 10. Am J Physiol Cell Physiol. 2015. PMID: 26063704 Free PMC article.
-
β3-adrenergic agonist counters oxidative stress and Na+-K+ pump inhibitory S-glutathionylation of placental cells: implications for preeclampsia.Am J Physiol Cell Physiol. 2025 Jan 1;328(1):C27-C39. doi: 10.1152/ajpcell.00379.2024. Epub 2024 Nov 4. Am J Physiol Cell Physiol. 2025. PMID: 39495253
-
Glutathionylation mediates angiotensin II-induced eNOS uncoupling, amplifying NADPH oxidase-dependent endothelial dysfunction.J Am Heart Assoc. 2014 Apr 22;3(2):e000731. doi: 10.1161/JAHA.113.000731. J Am Heart Assoc. 2014. PMID: 24755153 Free PMC article.
-
Endothelial Dysfunction: Is There a Hyperglycemia-Induced Imbalance of NOX and NOS?Int J Mol Sci. 2019 Aug 2;20(15):3775. doi: 10.3390/ijms20153775. Int J Mol Sci. 2019. PMID: 31382355 Free PMC article. Review.
-
Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling.Cardiovasc Res. 2009 Apr 1;82(1):9-20. doi: 10.1093/cvr/cvp031. Epub 2009 Jan 29. Cardiovasc Res. 2009. PMID: 19179352 Review.
Cited by
-
Therapeutic Potential for Beta-3 Adrenoreceptor Agonists in Peripheral Arterial Disease and Diabetic Foot Ulcers.Biomedicines. 2023 Nov 30;11(12):3187. doi: 10.3390/biomedicines11123187. Biomedicines. 2023. PMID: 38137408 Free PMC article. Review.
-
β3 adrenergic agonism: A novel pathway which improves right ventricular-pulmonary arterial hemodynamics in pulmonary arterial hypertension.Physiol Rep. 2023 Jan;11(1):e15549. doi: 10.14814/phy2.15549. Physiol Rep. 2023. PMID: 36597221 Free PMC article.
-
Nitric oxide signalling in cardiovascular health and disease.Nat Rev Cardiol. 2018 May;15(5):292-316. doi: 10.1038/nrcardio.2017.224. Epub 2018 Feb 1. Nat Rev Cardiol. 2018. PMID: 29388567 Review.
-
Improvement of Vascular Insulin Sensitivity by Ranolazine.Int J Mol Sci. 2023 Aug 31;24(17):13532. doi: 10.3390/ijms241713532. Int J Mol Sci. 2023. PMID: 37686345 Free PMC article.
-
Protocol for the Stimulating β3-Adrenergic Receptors for Peripheral Artery Disease (STAR-PAD) trial: a double-blinded, randomised, placebo-controlled study evaluating the effects of mirabegron on functional performance in patients with peripheral arterial disease.BMJ Open. 2021 Sep 28;11(9):e049858. doi: 10.1136/bmjopen-2021-049858. BMJ Open. 2021. PMID: 34588252 Free PMC article.
References
-
- Guzik TJ, Harrison DG. Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug Discovery Today. 2006;11:524–533. - PubMed
-
- Zimmet JM, Hare JM. Nitroso‐redox interactions in the cardiovascular system. Circulation. 2006;114:1531–1544. - PubMed
-
- Burgoyne JR, Mongue‐Din H, Eaton P, Shah AM. Redox signaling in cardiac physiology and pathology. Circ Res. 2012;111:1091–1106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

