Viral-mimicking protein nanoparticle vaccine for eliciting anti-tumor responses
- PMID: 26894870
- PMCID: PMC4775383
- DOI: 10.1016/j.biomaterials.2016.01.056
Viral-mimicking protein nanoparticle vaccine for eliciting anti-tumor responses
Abstract
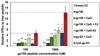
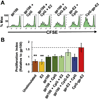
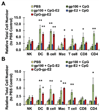
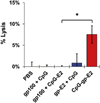
The immune system is a powerful resource for the eradication of cancer, but to overcome the low immunogenicity of tumor cells, a sufficiently strong CD8(+) T cell-mediated adaptive immune response is required. Nanoparticulate biomaterials represent a potentially effective delivery system for cancer vaccines, as they can be designed to mimic viruses, which are potent inducers of cellular immunity. We have been exploring the non-viral pyruvate dehydrogenase E2 protein nanoparticle as a biomimetic platform for cancer vaccine delivery. Simultaneous conjugation of a melanoma-associated gp100 epitope and CpG to the E2 nanoparticle (CpG-gp-E2) yielded an antigen-specific increase in the CD8(+) T cell proliferation index and IFN-γ secretion by 1.5-fold and 5-fold, respectively, compared to an unbound peptide and CpG formulation. Remarkably, a single nanoparticle immunization resulted in a 120-fold increase in the frequency of melanoma epitope-specific CD8(+) T cells in draining lymph nodes and a 30-fold increase in the spleen, relative to free peptide with free CpG. Furthermore, in the very aggressive B16 melanoma murine tumor model, prophylactic immunization with CpG-gp-E2 delayed the onset of tumor growth by approximately 5.5 days and increased animal survival time by approximately 40%, compared to PBS-treated animals. These results show that by combining optimal particle size and simultaneous co-delivery of molecular vaccine components, antigen-specific anti-tumor immune responses can be significantly increased.
Keywords: Biomimetic; CD8; Protein nanoparticle; T cells; Tumor-associated antigen; Vaccine.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Multi-compartmental vaccine delivery system for enhanced immune response to gp100 peptide antigen in melanoma immunotherapy.Pharm Res. 2012 Dec;29(12):3393-403. doi: 10.1007/s11095-012-0834-1. Epub 2012 Jul 18. Pharm Res. 2012. PMID: 22806408
-
Cytomegalovirus-Based Vaccine Expressing a Modified Tumor Antigen Induces Potent Tumor-Specific CD8(+) T-cell Response and Protects Mice from Melanoma.Cancer Immunol Res. 2015 May;3(5):536-46. doi: 10.1158/2326-6066.CIR-14-0044. Epub 2015 Jan 29. Cancer Immunol Res. 2015. PMID: 25633711
-
Targeted immunotherapy using reconstituted chaperone complexes of heat shock protein 110 and melanoma-associated antigen gp100.Cancer Res. 2003 May 15;63(10):2553-60. Cancer Res. 2003. PMID: 12750279
-
Cancer vaccines: Trafficking of tumor-specific T cells to tumor after therapeutic vaccination.Int J Biochem Cell Biol. 2014 Aug;53:46-50. doi: 10.1016/j.biocel.2014.04.019. Epub 2014 May 2. Int J Biochem Cell Biol. 2014. PMID: 24796845 Free PMC article. Review.
-
Designing therapeutic cancer vaccines by mimicking viral infections.Cancer Immunol Immunother. 2017 Feb;66(2):203-213. doi: 10.1007/s00262-016-1834-5. Epub 2016 Apr 6. Cancer Immunol Immunother. 2017. PMID: 27052572 Free PMC article. Review.
Cited by
-
Protein-based nanoparticles in cancer vaccine development.Nanomedicine. 2019 Jan;15(1):164-174. doi: 10.1016/j.nano.2018.09.004. Epub 2018 Oct 4. Nanomedicine. 2019. PMID: 30291897 Free PMC article. Review.
-
Delivery of Immunostimulatory Cargos in Nanocarriers Enhances Anti-Tumoral Nanovaccine Efficacy.Int J Mol Sci. 2023 Jul 29;24(15):12174. doi: 10.3390/ijms241512174. Int J Mol Sci. 2023. PMID: 37569548 Free PMC article. Review.
-
Orientation of Antigen Display on Self-Assembling Protein Nanoparticles Influences Immunogenicity.Vaccines (Basel). 2021 Jan 29;9(2):103. doi: 10.3390/vaccines9020103. Vaccines (Basel). 2021. PMID: 33572803 Free PMC article.
-
Identifying Key Drivers of Efficient B Cell Responses: On the Role of T Help, Antigen-Organization, and Toll-like Receptor Stimulation for Generating a Neutralizing Anti-Dengue Virus Response.Vaccines (Basel). 2024 Jun 14;12(6):661. doi: 10.3390/vaccines12060661. Vaccines (Basel). 2024. PMID: 38932390 Free PMC article.
-
Biomedical polymers: synthesis, properties, and applications.Sci China Chem. 2022;65(6):1010-1075. doi: 10.1007/s11426-022-1243-5. Epub 2022 Apr 24. Sci China Chem. 2022. PMID: 35505924 Free PMC article. Review.
References
-
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

