cGMP Signalling Mediates Water Sensation (Hydrosensation) and Hydrotaxis in Caenorhabditis elegans
- PMID: 26891989
- PMCID: PMC4759535
- DOI: 10.1038/srep19779
cGMP Signalling Mediates Water Sensation (Hydrosensation) and Hydrotaxis in Caenorhabditis elegans
Abstract
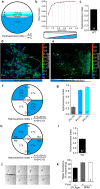
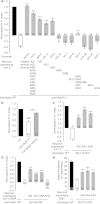
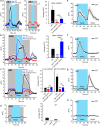
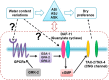
Animals have developed the ability to sense the water content in their habitats, including hygrosensation (sensing humidity in the air) and hydrosensation (sensing the water content in other microenvironments), and they display preferences for specific water contents that influence their mating, reproduction and geographic distribution. We developed and employed four quantitative behavioural test paradigms to investigate the molecular and cellular mechanisms underlying sensing the water content in an agar substrate (hydrosensation) and hydrotaxis in Caenorhabditis elegans. By combining a reverse genetic screen with genetic manipulation, optogenetic neuronal manipulation and in vivo Ca(2+) imaging, we demonstrate that adult worms avoid the wetter areas of agar plates and hypo-osmotic water droplets. We found that the cGMP signalling pathway in ciliated sensory neurons is involved in hydrosensation and hydrotaxis in Caenorhabditis elegans.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Humidity sensation requires both mechanosensory and thermosensory pathways in Caenorhabditis elegans.Proc Natl Acad Sci U S A. 2014 Jun 3;111(22):8269-74. doi: 10.1073/pnas.1322512111. Epub 2014 May 19. Proc Natl Acad Sci U S A. 2014. PMID: 24843133 Free PMC article.
-
Compartmentalized cGMP Responses of Olfactory Sensory Neurons in Caenorhabditis elegans.J Neurosci. 2017 Apr 5;37(14):3753-3763. doi: 10.1523/JNEUROSCI.2628-16.2017. Epub 2017 Mar 7. J Neurosci. 2017. PMID: 28270568 Free PMC article.
-
Humidity sensation, cockroaches, worms, and humans: are common sensory mechanisms for hygrosensation shared across species?J Neurophysiol. 2015 Aug;114(2):763-7. doi: 10.1152/jn.00730.2014. Epub 2014 Oct 15. J Neurophysiol. 2015. PMID: 25318766 Free PMC article.
-
How Caenorhabditis elegans Senses Mechanical Stress, Temperature, and Other Physical Stimuli.Genetics. 2019 May;212(1):25-51. doi: 10.1534/genetics.118.300241. Genetics. 2019. PMID: 31053616 Free PMC article. Review.
-
Sensory signaling in Caenorhabditis elegans.Curr Opin Neurobiol. 1996 Aug;6(4):494-9. doi: 10.1016/s0959-4388(96)80055-9. Curr Opin Neurobiol. 1996. PMID: 8794103 Review.
Cited by
-
GCY-35/GCY-36-TAX-2/TAX-4 Signalling in O2 Sensory Neurons Mediates Acute Functional Ethanol Tolerance in Caenorhabditis elegans.Sci Rep. 2018 Feb 14;8(1):3020. doi: 10.1038/s41598-018-20477-z. Sci Rep. 2018. PMID: 29445226 Free PMC article.
-
Electrotaxis-on-Chip to Quantify Neutrophil Migration Towards Electrochemical Gradients.Front Immunol. 2021 Aug 6;12:674727. doi: 10.3389/fimmu.2021.674727. eCollection 2021. Front Immunol. 2021. PMID: 34421891 Free PMC article.
-
Immediate activation of chemosensory neuron gene expression by bacterial metabolites is selectively induced by distinct cyclic GMP-dependent pathways in Caenorhabditis elegans.PLoS Genet. 2020 Aug 10;16(8):e1008505. doi: 10.1371/journal.pgen.1008505. eCollection 2020 Aug. PLoS Genet. 2020. PMID: 32776934 Free PMC article.
-
Wrapping culture plates with Parafilm M® increases Caenorhabditis elegans growth.BMC Res Notes. 2019 Dec 19;12(1):818. doi: 10.1186/s13104-019-4854-3. BMC Res Notes. 2019. PMID: 31856898 Free PMC article.
-
Reciprocal modulation of 5-HT and octopamine regulates pumping via feedforward and feedback circuits in C. elegans.Proc Natl Acad Sci U S A. 2019 Apr 2;116(14):7107-7112. doi: 10.1073/pnas.1819261116. Epub 2019 Mar 14. Proc Natl Acad Sci U S A. 2019. PMID: 30872487 Free PMC article.
References
-
- Liu L. et al. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature 450, 294–298 (2007). - PubMed
-
- Altner H. & Loftus R. Ultrastructure and Function of Insect Thermo- And Hygroreceptors. Ann Rev Entomol 30, 273–295 (1985).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

