A Novel Toll-Like Receptor 9 Agonist, MGN1703, Enhances HIV-1 Transcription and NK Cell-Mediated Inhibition of HIV-1-Infected Autologous CD4+ T Cells
- PMID: 26889036
- PMCID: PMC4836316
- DOI: 10.1128/JVI.00222-16
A Novel Toll-Like Receptor 9 Agonist, MGN1703, Enhances HIV-1 Transcription and NK Cell-Mediated Inhibition of HIV-1-Infected Autologous CD4+ T Cells
Abstract
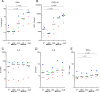
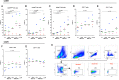
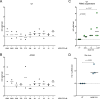
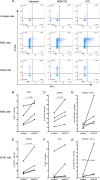
Toll-like receptor (TLR) agonists are potent enhancers of innate antiviral immunity and may also reverse HIV-1 latency. Therefore, TLR agonists have a potential role in the context of a "shock-and-kill" approach to eradicate HIV-1. Our extensive preclinical evaluation suggests that a novel TLR9 agonist, MGN1703, may indeed perform both functions in an HIV-1 eradication trial. Peripheral blood mononuclear cells (PBMCs) from aviremic HIV-1-infected donors on antiretroviral therapy (ART) that were incubated with MGN1703 ex vivo exhibited increased secretion of interferon alpha (IFN-α) (P= 0.005) and CXCL10 (P= 0.0005) in culture supernatants. Within the incubated PBMC pool, there were higher proportions of CD69-positive CD56(dim)CD16(+)NK cells (P= 0.001) as well as higher proportions of CD107a-positive (P= 0.002) and IFN-γ-producing (P= 0.038) NK cells. Incubation with MGN1703 also increased the proportions of CD69-expressing CD4(+)and CD8(+)T cells. Furthermore, CD4(+)T cells within the pool of MGN1703-incubated PBMCs showed enhanced levels of unspliced HIV-1 RNA (P= 0.036). Importantly, MGN1703 increased the capacity of NK cells to inhibit virus spread within a culture of autologous CD4(+)T cells assessed by using an HIV-1 p24 enzyme-linked immunosorbent assay (ELISA) (P= 0.03). In conclusion, we show that MGN1703 induced strong antiviral innate immune responses, enhanced HIV-1 transcription, and boosted NK cell-mediated suppression of HIV-1 infection in autologous CD4(+)T cells. These findings support clinical testing of MGN1703 in HIV-1 eradication trials.
Importance: We demonstrate that MGN1703 (a TLR9 agonist currently undergoing phase 3 clinical testing for the treatment of metastatic colorectal cancer) induces potent antiviral responses in immune effector cells from HIV-1-infected individuals on suppressive antiretroviral therapy. The significantly improved safety and tolerability profiles of MGN1703 versus TLR9 agonists of the CpG-oligodeoxynucleotide (CpG-ODN) family are due to its novel "dumbbell-shape" structure made of covalently closed, natural DNA. In our study, we found that incubation of peripheral blood mononuclear cells with MGN1703 results in natural killer cell activation and increased natural killer cell function, which significantly inhibited the spread of HIV in a culture of autologous CD4(+)T cells. Furthermore, we discovered that MGN1703-mediated activation can enhance HIV-1 transcription in CD4(+)T cells, suggesting that this molecule may serve a dual purpose in HIV-1 eradication therapy: enhanced immune function and latency reversal. These findings provide a strong preclinical basis for the inclusion of MGN1703 in an HIV eradication clinical trial.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
TLR1/2 Agonist Enhances Reversal of HIV-1 Latency and Promotes NK Cell-Induced Suppression of HIV-1-Infected Autologous CD4+ T Cells.J Virol. 2021 Aug 10;95(17):e0081621. doi: 10.1128/JVI.00816-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34133900 Free PMC article.
-
Short-Course Toll-Like Receptor 9 Agonist Treatment Impacts Innate Immunity and Plasma Viremia in Individuals With Human Immunodeficiency Virus Infection.Clin Infect Dis. 2017 Jun 15;64(12):1686-1695. doi: 10.1093/cid/cix201. Clin Infect Dis. 2017. PMID: 28329286 Free PMC article. Clinical Trial.
-
Interleukin-15-Stimulated Natural Killer Cells Clear HIV-1-Infected Cells following Latency Reversal Ex Vivo.J Virol. 2018 May 29;92(12):e00235-18. doi: 10.1128/JVI.00235-18. Print 2018 Jun 15. J Virol. 2018. PMID: 29593039 Free PMC article.
-
MGN1703, an immunomodulator and toll-like receptor 9 (TLR-9) agonist: from bench to bedside.Crit Rev Oncol Hematol. 2015 Apr;94(1):31-44. doi: 10.1016/j.critrevonc.2014.12.002. Epub 2014 Dec 19. Crit Rev Oncol Hematol. 2015. PMID: 25577571 Review.
-
Natural killer cells in HIV-1 infection and therapy.AIDS. 2017 Nov 13;31(17):2317-2330. doi: 10.1097/QAD.0000000000001645. AIDS. 2017. PMID: 28926399 Free PMC article. Review.
Cited by
-
Dual TLR2 and TLR7 agonists as HIV latency-reversing agents.JCI Insight. 2018 Oct 4;3(19):e122673. doi: 10.1172/jci.insight.122673. JCI Insight. 2018. PMID: 30282829 Free PMC article.
-
Boosting the Immune System for HIV Cure: A γδ T Cell Perspective.Front Cell Infect Microbiol. 2020 May 19;10:221. doi: 10.3389/fcimb.2020.00221. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32509594 Free PMC article. Review.
-
Pattern Recognition Receptor Ligands as an Emerging Therapeutic Agent for Latent HIV-1 Infection.Front Cell Infect Microbiol. 2020 May 8;10:216. doi: 10.3389/fcimb.2020.00216. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32457851 Free PMC article. Review.
-
In Vitro Exposure to Prostratin but Not Bryostatin-1 Improves Natural Killer Cell Functions Including Killing of CD4+ T Cells Harboring Reactivated Human Immunodeficiency Virus.Front Immunol. 2018 Jun 29;9:1514. doi: 10.3389/fimmu.2018.01514. eCollection 2018. Front Immunol. 2018. PMID: 30008723 Free PMC article.
-
Unraveling the Complex Interconnection between Specific Inflammatory Signaling Pathways and Mechanisms Involved in HIV-Associated Colorectal Oncogenesis.Cancers (Basel). 2023 Jan 25;15(3):748. doi: 10.3390/cancers15030748. Cancers (Basel). 2023. PMID: 36765706 Free PMC article. Review.
References
-
- Seay K, Church C, Zheng JH, Deneroff K, Ochsenbauer C, Kappes JC, Liu B, Jeng EK, Wong HC, Goldstein H. 2015. In vivo activation of human NK cells by treatment with an interleukin-15 superagonist potently inhibits acute in vivo HIV-1 infection in humanized mice. J Virol 89:6264–6274. doi:10.1128/JVI.00563-15. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

