Annexin A1 and the Resolution of Inflammation: Modulation of Neutrophil Recruitment, Apoptosis, and Clearance
- PMID: 26885535
- PMCID: PMC4738713
- DOI: 10.1155/2016/8239258
Annexin A1 and the Resolution of Inflammation: Modulation of Neutrophil Recruitment, Apoptosis, and Clearance
Abstract
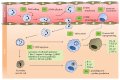
Neutrophils (also named polymorphonuclear leukocytes or PMN) are essential components of the immune system, rapidly recruited to sites of inflammation, providing the first line of defense against invading pathogens. Since neutrophils can also cause tissue damage, their fine-tuned regulation at the inflammatory site is required for proper resolution of inflammation. Annexin A1 (AnxA1), also known as lipocortin-1, is an endogenous glucocorticoid-regulated protein, which is able to counterregulate the inflammatory events restoring homeostasis. AnxA1 and its mimetic peptides inhibit neutrophil tissue accumulation by reducing leukocyte infiltration and activating neutrophil apoptosis. AnxA1 also promotes monocyte recruitment and clearance of apoptotic leukocytes by macrophages. More recently, some evidence has suggested the ability of AnxA1 to induce macrophage reprogramming toward a resolving phenotype, resulting in reduced production of proinflammatory cytokines and increased release of immunosuppressive and proresolving molecules. The combination of these mechanisms results in an effective resolution of inflammation, pointing to AnxA1 as a promising tool for the development of new therapeutic strategies to treat inflammatory diseases.
Figures
Similar articles
-
Inhibition of neutrophil and monocyte recruitment by endogenous and exogenous lipocortin 1.Br J Pharmacol. 1997 Mar;120(6):1075-82. doi: 10.1038/sj.bjp.0701029. Br J Pharmacol. 1997. PMID: 9134220 Free PMC article.
-
Annexin A1 promotes timely resolution of inflammation in murine gout.Eur J Immunol. 2017 Mar;47(3):585-596. doi: 10.1002/eji.201646551. Epub 2017 Jan 12. Eur J Immunol. 2017. PMID: 27995621
-
The resolution of acute inflammation induced by cyclic AMP is dependent on annexin A1.J Biol Chem. 2017 Aug 18;292(33):13758-13773. doi: 10.1074/jbc.M117.800391. Epub 2017 Jun 27. J Biol Chem. 2017. PMID: 28655761 Free PMC article.
-
The advantageous role of annexin A1 in cardiovascular disease.Cell Adh Migr. 2017 May 4;11(3):261-274. doi: 10.1080/19336918.2016.1259059. Epub 2016 Nov 18. Cell Adh Migr. 2017. PMID: 27860536 Free PMC article. Review.
-
The resolution of inflammation: Principles and challenges.Semin Immunol. 2015 May;27(3):149-60. doi: 10.1016/j.smim.2015.03.014. Epub 2015 Apr 22. Semin Immunol. 2015. PMID: 25911383 Review.
Cited by
-
Neutrophil-mediated inflammation in the pathogenesis of Clostridium difficile infections.Anaerobe. 2016 Oct;41:85-90. doi: 10.1016/j.anaerobe.2016.04.001. Epub 2016 Apr 5. Anaerobe. 2016. PMID: 27063896 Free PMC article. Review.
-
Mechanisms and Clinical Applications of Glucocorticoid Steroids in Muscular Dystrophy.J Neuromuscul Dis. 2021;8(1):39-52. doi: 10.3233/JND-200556. J Neuromuscul Dis. 2021. PMID: 33104035 Free PMC article. Review.
-
Humanized Mouse Models of Systemic Lupus Erythematosus: Opportunities and Challenges.Front Immunol. 2022 Jan 18;12:816956. doi: 10.3389/fimmu.2021.816956. eCollection 2021. Front Immunol. 2022. PMID: 35116040 Free PMC article. Review.
-
Quo Vadis Anesthesiologist? The Value Proposition of Future Anesthesiologists Lies in Preserving or Restoring Presurgical Health after Surgical Insult.J Clin Med. 2022 Feb 21;11(4):1135. doi: 10.3390/jcm11041135. J Clin Med. 2022. PMID: 35207406 Free PMC article.
-
ANNEXIN A1: Roles in Placenta, Cell Survival, and Nucleus.Cells. 2022 Jun 29;11(13):2057. doi: 10.3390/cells11132057. Cells. 2022. PMID: 35805141 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

