T-cell epitope conservation across allergen species is a major determinant of immunogenicity
- PMID: 26883464
- PMCID: PMC4975972
- DOI: 10.1016/j.jaci.2015.11.034
T-cell epitope conservation across allergen species is a major determinant of immunogenicity
Abstract
Background: Patients with pollen allergies are frequently polysensitized. Pollens contain epitopes that are conserved across multiple species.
Objective: We sought to demonstrate that cross-reactive T cells that recognize conserved epitopes show higher levels of expansion than T cells recognizing monospecific epitopes because of more frequent stimulation.
Method: RNA was sequenced from 9 pollens, and the reads were assembled de novo into more than 50,000 transcripts. T-cell epitopes from timothy grass (Phleum pratense) were examined for conservation in these transcripts, and this was correlated to their ability to induce T-cell responses. T cells were expanded in vitro with P pratense-derived peptides and tested for cross-reactivity to pollen extracts in ELISpot assays.
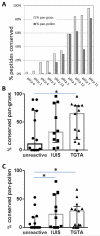
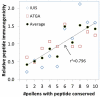
Results: We found that antigenic proteins are more conserved than nonimmunogenic proteins in P pratense pollen. Additionally, P pratense epitopes that were highly conserved across pollens elicited more T-cell responses in donors with grass allergy than less conserved epitopes. Moreover, conservation of a P pratense peptide at the transcriptomic level correlated with the ability of that peptide to trigger T cells that were cross-reactive with other non-P pratense pollen extracts.
Conclusion: We found a correlation between conservation of peptides in plant pollens and their T-cell immunogenicity within P pratense, as well as their ability to induce cross-reactive T-cell responses. T cells recognizing conserved epitopes might be more prominent because they can be stimulated by a broader range of pollens and thereby drive polysensitization in allergic donors. We propose that conserved peptides could potentially be used in diagnostic or immunomodulatory approaches that address the issue of polysensitization and target multiple pollen allergies.
Keywords: RNA sequencing; T cell; cross-reactivity; epitope; pollen allergy; sequence conservation; timothy grass allergy; transcriptome.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Reply.J Allergy Clin Immunol. 2016 Oct;138(4):1237-1238. doi: 10.1016/j.jaci.2016.04.054. Epub 2016 Jul 30. J Allergy Clin Immunol. 2016. PMID: 27484034 No abstract available.
-
T-cell receptor-mediated cross-reactivity to different allergens is driven by recognition of homologous, phylogenetically conserved epitopes.J Allergy Clin Immunol. 2016 Oct;138(4):1237. doi: 10.1016/j.jaci.2016.04.055. Epub 2016 Jul 30. J Allergy Clin Immunol. 2016. PMID: 27484036 No abstract available.
Similar articles
-
Grass-specific CD4(+) T-cells exhibit varying degrees of cross-reactivity, implications for allergen-specific immunotherapy.Clin Exp Allergy. 2014 Jul;44(7):986-98. doi: 10.1111/cea.12324. Clin Exp Allergy. 2014. PMID: 24708411 Free PMC article.
-
Sequence conservation predicts T cell reactivity against ragweed allergens.Clin Exp Allergy. 2016 Sep;46(9):1194-205. doi: 10.1111/cea.12772. Epub 2016 Jul 26. Clin Exp Allergy. 2016. PMID: 27359111 Free PMC article.
-
Unique and cross-reactive T cell epitope peptides of the major Bahia grass pollen allergen, Pas n 1.Int Arch Allergy Immunol. 2012;159(4):355-66. doi: 10.1159/000338290. Epub 2012 Jul 25. Int Arch Allergy Immunol. 2012. PMID: 22832594
-
Characteristics and immunobiology of grass pollen allergens.Int Arch Allergy Immunol. 2003 Feb;130(2):87-107. doi: 10.1159/000069013. Int Arch Allergy Immunol. 2003. PMID: 12673063 Review.
-
Multiple grass mixes as opposed to single grasses for allergen immunotherapy in allergic rhinitis.Clin Exp Allergy. 2013 Nov;43(11):1202-16. doi: 10.1111/cea.12128. Clin Exp Allergy. 2013. PMID: 24152153 Free PMC article. Review.
Cited by
-
A Review on T Cell Epitopes Identified Using Prediction and Cell-Mediated Immune Models for Mycobacterium tuberculosis and Bordetella pertussis.Front Immunol. 2018 Nov 29;9:2778. doi: 10.3389/fimmu.2018.02778. eCollection 2018. Front Immunol. 2018. PMID: 30555469 Free PMC article. Review.
-
T Cell Epitope Predictions.Annu Rev Immunol. 2020 Apr 26;38:123-145. doi: 10.1146/annurev-immunol-082119-124838. Epub 2020 Feb 11. Annu Rev Immunol. 2020. PMID: 32045313 Free PMC article. Review.
-
Experimental validation of the RATE tool for inferring HLA restrictions of T cell epitopes.BMC Immunol. 2017 Jun 21;18(Suppl 1):20. doi: 10.1186/s12865-017-0204-1. BMC Immunol. 2017. PMID: 28681704 Free PMC article.
-
Hydrolyzed diets may stimulate food-reactive lymphocytes in dogs.J Vet Med Sci. 2020 Feb 18;82(2):177-183. doi: 10.1292/jvms.19-0222. Epub 2019 Dec 25. J Vet Med Sci. 2020. PMID: 31875597 Free PMC article.
-
Microbiota epitope similarity either dampens or enhances the immunogenicity of disease-associated antigenic epitopes.PLoS One. 2018 May 7;13(5):e0196551. doi: 10.1371/journal.pone.0196551. eCollection 2018. PLoS One. 2018. PMID: 29734356 Free PMC article.
References
-
- Weber RW. Cross-reactivity of pollen allergens. Current allergy and asthma reports. 2004;4:401–408. - PubMed
-
- Martinez-Cocera C, et al. Immunotherapy with a Phleum pratense allergen extract induces an immune response to a grass-mix allergen extract. Journal of investigational allergology & clinical immunology. 2010;20:13–19. - PubMed
-
- Hejl C, et al. Phleum pratense alone is sufficient for allergen-specific immunotherapy against allergy to Pooideae grass pollens. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2009;39:752–759. - PubMed
-
- Palomares O, et al. Role of Treg in immune regulation of allergic diseases. European journal of immunology. 2010;40:1232–1240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

