Expression profile and overexpression outcome indicate a role for βKlotho in skeletal muscle fibro/adipogenesis
- PMID: 26881702
- PMCID: PMC5070976
- DOI: 10.1111/febs.13682
Expression profile and overexpression outcome indicate a role for βKlotho in skeletal muscle fibro/adipogenesis
Abstract
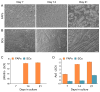
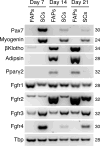
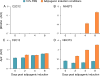
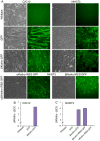
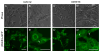
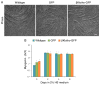
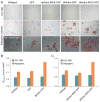
Regeneration of skeletal muscles is required throughout life to ensure optimal performance. Therefore, a better understanding of the resident cells involved in muscle repair is essential. Muscle repair relies on satellite cells (SCs), the resident myogenic progenitors, but also involves the contribution of interstitial cells including fibro/adipocyte progenitors (FAPs). To elucidate the role of the fibroblast growth factor (FGF) signaling in these two cell populations, we previously analyzed freshly isolated cells for their FGF receptor (FGFR) signature. Transcript analysis of the four Fgfr genes revealed distinct expression profiles for SCs and FAPs, raising the possibility that these two cell types have different FGF-mediated processes. Here, we pursued this hypothesis exploring the role of the Klotho genes, whose products are known to function as FGFR co-receptors for the endocrine FGF subfamily. Isolated SC and FAP populations were analyzed in culture, exhibiting spontaneous myogenic or adipogenic differentiation, respectively. αKlotho expression was not detected in either population. βKlotho expression, while not detected in SCs, was strongly upregulated in FAPs entering adipogenic differentiation, coinciding with expression of a panel of adipogenic genes and preceding the appearance of intracellular lipid droplets. Overexpression of βKlotho in mouse cell line models enhanced adipogenesis in NIH3T3 fibroblasts but had no effect on C2C12 myogenic cells. Our study supports a pro-adipogenic role for βKlotho in skeletal muscle fibro/adipogenesis and calls for further research on involvement of the FGF-FGFR-βKlotho axis in the fibro/adipogenic infiltration associated with functional deterioration of skeletal muscle in aging and muscular dystrophy.
Keywords: FAPs; FGF21; PiggyBac transposon; adipogenesis; adipogenic differentiation; fibro/adipocytes; fibroblast growth factor receptor; satellite cells; αKlotho; βKlotho.
© 2016 Federation of European Biochemical Societies.
Figures

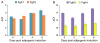
Similar articles
-
betaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c.Mol Endocrinol. 2008 Apr;22(4):1006-14. doi: 10.1210/me.2007-0313. Epub 2008 Jan 10. Mol Endocrinol. 2008. PMID: 18187602 Free PMC article.
-
Selective activation of FGFR4 by an FGF19 variant does not improve glucose metabolism in ob/ob mice.Proc Natl Acad Sci U S A. 2009 Aug 25;106(34):14379-84. doi: 10.1073/pnas.0907812106. Epub 2009 Aug 12. Proc Natl Acad Sci U S A. 2009. PMID: 19706524 Free PMC article.
-
Characterization of the Skeletal Muscle Secretome Reveals a Role for Extracellular Vesicles and IL1α/IL1β in Restricting Fibro/Adipogenic Progenitor Adipogenesis.Biomolecules. 2021 Aug 8;11(8):1171. doi: 10.3390/biom11081171. Biomolecules. 2021. PMID: 34439837 Free PMC article.
-
Signaling pathways regulating the fate of fibro/adipogenic progenitors (FAPs) in skeletal muscle regeneration and disease.FEBS J. 2022 Nov;289(21):6484-6517. doi: 10.1111/febs.16080. Epub 2021 Jul 6. FEBS J. 2022. PMID: 34143565 Review.
-
Fibro-adipogenic progenitors in skeletal muscle homeostasis, regeneration and diseases.Open Biol. 2021 Dec;11(12):210110. doi: 10.1098/rsob.210110. Epub 2021 Dec 8. Open Biol. 2021. PMID: 34875199 Free PMC article. Review.
Cited by
-
CD34 regulates the skeletal muscle response to hypoxia.J Muscle Res Cell Motil. 2019 Dec;40(3-4):309-318. doi: 10.1007/s10974-019-09525-x. Epub 2019 Jun 20. J Muscle Res Cell Motil. 2019. PMID: 31222587
-
Metabolites related to renal function, immune activation, and carbamylation are associated with muscle composition in older adults.Exp Gerontol. 2017 Dec 15;100:1-10. doi: 10.1016/j.exger.2017.10.003. Epub 2017 Oct 10. Exp Gerontol. 2017. PMID: 29030163 Free PMC article.
-
Fibroblast growth factor 23 does not directly influence skeletal muscle cell proliferation and differentiation or ex vivo muscle contractility.Am J Physiol Endocrinol Metab. 2018 Oct 1;315(4):E594-E604. doi: 10.1152/ajpendo.00343.2017. Epub 2018 Mar 20. Am J Physiol Endocrinol Metab. 2018. PMID: 29558205 Free PMC article.
-
Exercise enhances skeletal muscle regeneration by promoting senescence in fibro-adipogenic progenitors.Nat Commun. 2020 Feb 14;11(1):889. doi: 10.1038/s41467-020-14734-x. Nat Commun. 2020. PMID: 32060352 Free PMC article.
-
Gait disturbances and muscle dysfunction in fibroblast growth factor 2 knockout mice.Sci Rep. 2021 May 26;11(1):11005. doi: 10.1038/s41598-021-90565-0. Sci Rep. 2021. PMID: 34040128 Free PMC article.
References
-
- Montarras D, L’Honoré A, Buckingham M. Lying low but ready for action: the quiescent muscle satellite cell. FEBS J. 2013;280:4036–4050. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

