Amyloid-β peptides in interaction with raft-mime model membranes: a neutron reflectivity insight
- PMID: 26880066
- PMCID: PMC4754687
- DOI: 10.1038/srep20997
Amyloid-β peptides in interaction with raft-mime model membranes: a neutron reflectivity insight
Abstract
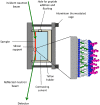
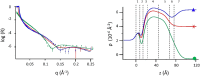
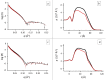
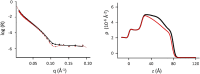
The role of first-stage β-amyloid aggregation in the development of the Alzheimer disease, is widely accepted but still unclear. Intimate interaction with the cell membrane is invoked. We designed Neutron Reflectometry experiments to reveal the existence and extent of the interaction between β-amyloid (Aβ) peptides and a lone customized biomimetic membrane, and their dependence on the aggregation state of the peptide. The membrane, asymmetrically containing phospholipids, GM1 and cholesterol in biosimilar proportion, is a model for a raft, a putative site for amyloid-cell membrane interaction. We found that the structured-oligomer of Aβ(1-42), its most acknowledged membrane-active state, is embedded as such into the external leaflet of the membrane. Conversely, the Aβ(1-42) unstructured early-oligomers deeply penetrate the membrane, likely mimicking the interaction at neuronal cell surfaces, when the Aβ(1-42) is cleaved from APP protein and the membrane constitutes a template for its further structural evolution. Moreover, the smaller Aβ(1-6) fragment, the N-terminal portion of Aβ, was also used. Aβ N-terminal is usually considered as involved in oligomer stabilization but not in the peptide-membrane interaction. Instead, it was seen to remove lipids from the bilayer, thus suggesting its role, once in the whole peptide, in membrane leakage, favouring peptide recruitment.
Figures

Similar articles
-
High-Affinity Binding of Monomeric but Not Oligomeric Amyloid-β to Ganglioside GM1 Containing Nanodiscs.Biochemistry. 2016 Dec 6;55(48):6662-6672. doi: 10.1021/acs.biochem.6b00829. Epub 2016 Nov 18. Biochemistry. 2016. PMID: 27933798
-
Effects of membrane interaction and aggregation of amyloid β-peptide on lipid mobility and membrane domain structure.Phys Chem Chem Phys. 2013 Jun 21;15(23):8929-39. doi: 10.1039/c3cp44517h. Epub 2013 Mar 21. Phys Chem Chem Phys. 2013. PMID: 23515399
-
Amyloid-β Interactions with Lipid Rafts in Biomimetic Systems: A Review of Laboratory Methods.Methods Mol Biol. 2021;2187:47-86. doi: 10.1007/978-1-0716-0814-2_4. Methods Mol Biol. 2021. PMID: 32770501 Review.
-
The effect of Alzheimer's Aβ aggregation state on the permeation of biomimetic lipid vesicles.Langmuir. 2010 Nov 16;26(22):17260-8. doi: 10.1021/la101581g. Epub 2010 Oct 5. Langmuir. 2010. PMID: 20923185
-
Interaction of Alzheimer's β-amyloid peptides with cholesterol: mechanistic insights into amyloid pore formation.Biochemistry. 2014 Jul 22;53(28):4489-502. doi: 10.1021/bi500373k. Epub 2014 Jul 11. Biochemistry. 2014. PMID: 25000142 Review.
Cited by
-
Structural features and lipid binding domain of tubulin on biomimetic mitochondrial membranes.Proc Natl Acad Sci U S A. 2017 May 2;114(18):E3622-E3631. doi: 10.1073/pnas.1619806114. Epub 2017 Apr 18. Proc Natl Acad Sci U S A. 2017. PMID: 28420794 Free PMC article.
-
Alzheimer's Disease as a Membrane Disorder: Spatial Cross-Talk Among Beta-Amyloid Peptides, Nicotinic Acetylcholine Receptors and Lipid Rafts.Front Cell Neurosci. 2019 Jul 18;13:309. doi: 10.3389/fncel.2019.00309. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31379503 Free PMC article. Review.
-
Sitosterol and glucosylceramide cooperative transversal and lateral uneven distribution in plant membranes.Sci Rep. 2021 Nov 3;11(1):21618. doi: 10.1038/s41598-021-00696-7. Sci Rep. 2021. PMID: 34732753 Free PMC article.
-
The Role of Ceramide and Sphingosine-1-Phosphate in Alzheimer's Disease and Other Neurodegenerative Disorders.Mol Neurobiol. 2019 Aug;56(8):5436-5455. doi: 10.1007/s12035-018-1448-3. Epub 2019 Jan 5. Mol Neurobiol. 2019. PMID: 30612333 Free PMC article. Review.
-
Human Lipoproteins at Model Cell Membranes: Effect of Lipoprotein Class on Lipid Exchange.Sci Rep. 2017 Aug 7;7(1):7478. doi: 10.1038/s41598-017-07505-0. Sci Rep. 2017. PMID: 28785025 Free PMC article.
References
-
- Small D. H. & Cappai R. A. Alzheimer and Alzheimer’s Disease: a Centennial Perspective. J. Neurochem. 99, 708–710 (2006). - PubMed
-
- Golde T. E., Dickson D. & Hutton M. Filling the Gaps in the Aβ Cascade Hypothesis of Alzheimer’s Disease. Curr. Alzheimer Res. 3, 421–430 (2006). - PubMed
-
- Kayed R. et al. Permeabilization of Lipid Bilayers Is a Common Conformation-dependent Activity of Soluble Amyloid Oligomers in Protein Misfolding Diseases. J. Biol. Chem.. 279, 46363–46366 (2004). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

