Intrinsic mechanical behavior of femoral cortical bone in young, osteoporotic and bisphosphonate-treated individuals in low- and high energy fracture conditions
- PMID: 26879146
- PMCID: PMC4754644
- DOI: 10.1038/srep21072
Intrinsic mechanical behavior of femoral cortical bone in young, osteoporotic and bisphosphonate-treated individuals in low- and high energy fracture conditions
Abstract
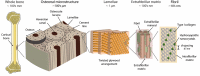
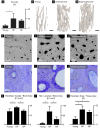
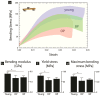
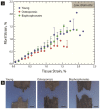
Bisphosphonates are a common treatment to reduce osteoporotic fractures. This treatment induces osseous structural and compositional changes accompanied by positive effects on osteoblasts and osteocytes. Here, we test the hypothesis that restored osseous cell behavior, which resembles characteristics of younger, healthy cortical bone, leads to improved bone quality. Microarchitecture and mechanical properties of young, treatment-naïve osteoporosis, and bisphosphonate-treated cases were investigated in femoral cortices. Tissue strength was measured using three-point bending. Collagen fibril-level deformation was assessed in non-traumatic and traumatic fracture states using synchrotron small-angle x-ray scattering (SAXS) at low and high strain rates. The lower modulus, strength and fibril deformation measured at low strain rates reflects susceptibility for osteoporotic low-energy fragility fractures. Independent of age, disease and treatment status, SAXS revealed reduced fibril plasticity at high strain rates, characteristic of traumatic fracture. The significantly reduced mechanical integrity in osteoporosis may originate from porosity and alterations to the intra/extrafibrillar structure, while the fibril deformation under treatment indicates improved nano-scale characteristics. In conclusion, losses in strength and fibril deformation at low strain rates correlate with the occurrence of fragility fractures in osteoporosis, while improvements in structural and mechanical properties following bisphosphonate treatment may foster resistance to fracture during physiological strain rates.
Figures
Similar articles
-
Intravenous treatment with ibandronate normalizes bone matrix mineralization and reduces cortical porosity after two years in male osteoporosis: a paired biopsy study.J Bone Miner Res. 2014 Feb;29(2):440-9. doi: 10.1002/jbmr.2035. J Bone Miner Res. 2014. PMID: 23832525 Clinical Trial.
-
The inferomedial femoral neck is compromised by age but not disease: Fracture toughness and the multifactorial mechanisms comprising reference point microindentation.J Mech Behav Biomed Mater. 2017 Nov;75:399-412. doi: 10.1016/j.jmbbm.2017.06.036. Epub 2017 Jun 30. J Mech Behav Biomed Mater. 2017. PMID: 28803114 Free PMC article.
-
Multiscale alterations in bone matrix quality increased fragility in steroid induced osteoporosis.Bone. 2016 Mar;84:15-24. doi: 10.1016/j.bone.2015.11.019. Epub 2015 Dec 2. Bone. 2016. PMID: 26657825 Free PMC article.
-
Cortical Bone Porosity: What Is It, Why Is It Important, and How Can We Detect It?Curr Osteoporos Rep. 2016 Oct;14(5):187-98. doi: 10.1007/s11914-016-0319-y. Curr Osteoporos Rep. 2016. PMID: 27623679 Review.
-
Biomechanics of osteoporotic fractures.Injury. 2007 Sep;38 Suppl 3:S69-76. doi: 10.1016/j.injury.2007.08.014. Injury. 2007. PMID: 17723795 Review.
Cited by
-
Mechanical Characterization of Bone: State of the Art in Experimental Approaches-What Types of Experiments Do People Do and How Does One Interpret the Results?Curr Osteoporos Rep. 2018 Aug;16(4):423-433. doi: 10.1007/s11914-018-0454-8. Curr Osteoporos Rep. 2018. PMID: 29915968 Free PMC article. Review.
-
The Effect of Formalin Preservation Time and Temperature on the Material Properties of Bovine Femoral Cortical Bone Tissue.Ann Biomed Eng. 2019 Apr;47(4):937-952. doi: 10.1007/s10439-019-02197-1. Epub 2019 Jan 22. Ann Biomed Eng. 2019. PMID: 30671755
-
Effect of non-enzymatic glycation on collagen nanoscale mechanisms in diabetic and age-related bone fragility.Biocell. 2023 Jun 21;47(7):1651-1659. doi: 10.32604/biocell.2023.028014. Biocell. 2023. PMID: 37693278 Free PMC article.
-
Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance.Proc Natl Acad Sci U S A. 2017 Aug 15;114(33):8722-8727. doi: 10.1073/pnas.1704460114. Epub 2017 Jul 31. Proc Natl Acad Sci U S A. 2017. PMID: 28760963 Free PMC article.
-
Long-term effects of bisphosphonate therapy: perforations, microcracks and mechanical properties.Sci Rep. 2017 Mar 6;7:43399. doi: 10.1038/srep43399. Sci Rep. 2017. PMID: 28262693 Free PMC article.
References
-
- Busse B. et al. Vitamin D deficiency induces early signs of aging in human bone, increasing the risk of fracture. Sci. Transl. Med. 5, 193ra88 (2013). - PubMed
-
- Zimmermann E. A. et al. Modifications to nano- and microstructural quality and the effects on mechanical integrity in Paget’s disease of bone. J. Bone Miner. Res. 30, 264–273 (2015). - PubMed
-
- Seeley D. G. et al. Which fractures are associated with low appendicular bone mass in elderly women? Ann. Intern. Med. 115, 837–842 (1991). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

