Principles of Virus Uncoating: Cues and the Snooker Ball
- PMID: 26875443
- PMCID: PMC7169695
- DOI: 10.1111/tra.12387
Principles of Virus Uncoating: Cues and the Snooker Ball
Abstract
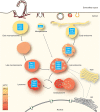
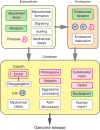
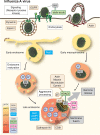
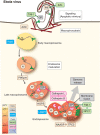
Viruses are spherical or complex shaped carriers of proteins, nucleic acids and sometimes lipids and sugars. They are metastable and poised for structural changes. These features allow viruses to communicate with host cells during entry, and to release the viral genome, a process known as uncoating. Studies have shown that hundreds of host factors directly or indirectly support this process. The cell provides molecules that promote stepwise virus uncoating, and direct the virus to the site of replication. It acts akin to a snooker player who delivers accurate and timely shots (cues) to the ball (virus) to score. The viruses, on the other hand, trick (snooker) the host, hijack its homeostasis systems, and dampen innate immune responses directed against danger signals. In this review, we discuss how cellular cues, facilitators, and built-in viral mechanisms promote uncoating. Cues come from receptors, enzymes and chemicals that act directly on the virus particle to alter its structure, trafficking and infectivity. Facilitators are defined as host factors that are involved in processes which indirectly enhance entry or uncoating. Unraveling the mechanisms of virus uncoating will continue to enhance understanding of cell functions, and help counteracting infections with chemicals and vaccines.
Keywords: cytoskeleton; endocytosis; low pH; membrane fusion; molecular motor; nuclear import; nuclear pore complex; penetration; signaling; virus structure.
© 2016 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures
Similar articles
-
Single HIV-1 Imaging Reveals Progression of Infection through CA-Dependent Steps of Docking at the Nuclear Pore, Uncoating, and Nuclear Transport.Cell Host Microbe. 2018 Apr 11;23(4):536-548.e6. doi: 10.1016/j.chom.2018.03.009. Cell Host Microbe. 2018. PMID: 29649444 Free PMC article.
-
DNA virus uncoating.Virology. 2015 May;479-480:578-90. doi: 10.1016/j.virol.2015.01.024. Epub 2015 Feb 26. Virology. 2015. PMID: 25728300 Review.
-
Dengue Virus Genome Uncoating Requires Ubiquitination.mBio. 2016 Jun 28;7(3):e00804-16. doi: 10.1128/mBio.00804-16. mBio. 2016. PMID: 27353759 Free PMC article.
-
Stepwise priming by acidic pH and a high K+ concentration is required for efficient uncoating of influenza A virus cores after penetration.J Virol. 2014 Nov;88(22):13029-46. doi: 10.1128/JVI.01430-14. Epub 2014 Aug 27. J Virol. 2014. PMID: 25165113 Free PMC article.
-
Influenza A virus uncoating.Adv Virus Res. 2020;106:1-38. doi: 10.1016/bs.aivir.2020.01.001. Epub 2020 Feb 13. Adv Virus Res. 2020. PMID: 32327145 Review.
Cited by
-
Mucolipin-2 Cation Channel Increases Trafficking Efficiency of Endocytosed Viruses.mBio. 2018 Jan 30;9(1):e02314-17. doi: 10.1128/mBio.02314-17. mBio. 2018. PMID: 29382735 Free PMC article.
-
Out of the ESCPE room: Emerging roles of endosomal SNX-BARs in receptor transport and host-pathogen interaction.Traffic. 2023 Jun;24(6):234-250. doi: 10.1111/tra.12885. Epub 2023 Apr 23. Traffic. 2023. PMID: 37089068 Free PMC article. Review.
-
The Anti-Infectious Role of Sphingosine in Microbial Diseases.Cells. 2021 May 4;10(5):1105. doi: 10.3390/cells10051105. Cells. 2021. PMID: 34064516 Free PMC article. Review.
-
Editorial: An expanded view of viruses.FEMS Microbiol Rev. 2017 Jan;41(1):1-4. doi: 10.1093/femsre/fuw044. FEMS Microbiol Rev. 2017. PMID: 28087690 Free PMC article. No abstract available.
-
A Message from the Human Placenta: Structural and Immunomodulatory Defense against SARS-CoV-2.Cells. 2020 Jul 25;9(8):1777. doi: 10.3390/cells9081777. Cells. 2020. PMID: 32722449 Free PMC article. Review.
References
-
- Mercer J, Schelhaas M, Helenius A. Virus entry by endocytosis. Annu Rev Biochem 2010;79:803–833. - PubMed
-
- Greber UF, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell 1993;75:477–486. - PubMed
-
- Greber UF, Singh I, Helenius A. Mechanisms of virus uncoating. Trends Microbiol 1994;2:52–56. - PubMed
-
- Yamauchi Y, Helenius A. Virus entry at a glance. J Cell Sci 2013;126:1289–1295. - PubMed
-
- Wolfrum N, Greber UF. Adenovirus signalling in entry. Cell Microbiol 2013;15:53–62. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

