Cytotoxic Potential of Bacillus cereus Strains ATCC 11778 and 14579 Against Human Lung Epithelial Cells Under Microaerobic Growth Conditions
- PMID: 26870026
- PMCID: PMC4735842
- DOI: 10.3389/fmicb.2016.00069
Cytotoxic Potential of Bacillus cereus Strains ATCC 11778 and 14579 Against Human Lung Epithelial Cells Under Microaerobic Growth Conditions
Abstract
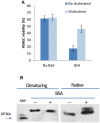
Bacillus cereus, a food poisoning bacterium closely related to Bacillus anthracis, secretes a multitude of virulence factors including enterotoxins, hemolysins, and phospholipases. However, the majority of the in vitro experiments evaluating the cytotoxic potential of B. cereus were carried out in the conditions of aeration, and the impact of the oxygen limitation in conditions encountered by the microbe in natural environment such as gastrointestinal tract remains poorly understood. This research reports comparative analysis of ATCC strains 11778 (BC1) and 14579 (BC2) in aerobic and microaerobic (static) cultures with regard to their toxicity for human lung epithelial cells. We showed that BC1 increased its toxicity upon oxygen limitation while BC2 was highly cytotoxic in both growth conditions. The combined effect of the pore-forming, cholesterol-dependent hemolysin, cereolysin O (CLO), and metabolic product(s) such as succinate produced in microaerobic conditions provided substantial contribution to the toxicity of BC1 but not BC2 which relied mainly on other toxins. This mechanism is shared between CB1 and B. anthracis. It involves the permeabilization of the cell membrane which facilitates transport of toxic bacterial metabolites into the cell. The toxicity of BC1 was potentiated in the presence of bovine serum albumin which appeared to serve as reservoir for bacteria-derived nitric oxide participating in the downstream production of reactive oxidizing species with the properties of peroxynitrite. In agreement with this the BC1 cultures demonstrated the increased oxidation of the indicator dye Amplex Red catalyzed by peroxidase as well as the increased toxicity in the presence of externally added ascorbic acid.
Keywords: Bacillus cereus; cereolysin O; culture filtrates; cytotoxicity; lung epithelial cells.
Figures
Similar articles
-
Anthrolysin O and fermentation products mediate the toxicity of Bacillus anthracis to lung epithelial cells under microaerobic conditions.FEMS Immunol Med Microbiol. 2011 Feb;61(1):15-27. doi: 10.1111/j.1574-695X.2010.00740.x. Epub 2010 Oct 14. FEMS Immunol Med Microbiol. 2011. PMID: 20946354 Free PMC article.
-
Toxigenic potential and heat survival of spore-forming bacteria isolated from bread and ingredients.Int J Food Microbiol. 2015 Mar 16;197:30-9. doi: 10.1016/j.ijfoodmicro.2014.12.017. Epub 2014 Dec 18. Int J Food Microbiol. 2015. PMID: 25555227
-
The impact of oxygen availability on stress survival and radical formation of Bacillus cereus.Int J Food Microbiol. 2009 Nov 15;135(3):303-11. doi: 10.1016/j.ijfoodmicro.2009.09.002. Epub 2009 Sep 9. Int J Food Microbiol. 2009. PMID: 19762101
-
The effect of selected factors on the survival of Bacillus cereus in the human gastrointestinal tract.Microb Pathog. 2015 May;82:7-14. doi: 10.1016/j.micpath.2015.03.015. Epub 2015 Mar 17. Microb Pathog. 2015. PMID: 25794697 Review.
-
Multifaceted toxin profile, an approach toward a better understanding of probiotic Bacillus cereus.Crit Rev Toxicol. 2019 Apr;49(4):342-356. doi: 10.1080/10408444.2019.1609410. Epub 2019 May 22. Crit Rev Toxicol. 2019. PMID: 31116061 Review.
Cited by
-
Probiotic Bacillus cereus Strains, a Potential Risk for Public Health in China.Front Microbiol. 2016 May 23;7:718. doi: 10.3389/fmicb.2016.00718. eCollection 2016. Front Microbiol. 2016. PMID: 27242738 Free PMC article.
-
Lactobacillus rhamnosus GR-1 attenuates foodborne Bacillus cereus-induced NLRP3 inflammasome activity in bovine mammary epithelial cells by protecting intercellular tight junctions.J Anim Sci Biotechnol. 2022 Sep 9;13(1):101. doi: 10.1186/s40104-022-00752-w. J Anim Sci Biotechnol. 2022. PMID: 36076276 Free PMC article.
-
Advanced Proteomics as a Powerful Tool for Studying Toxins of Human Bacterial Pathogens.Toxins (Basel). 2019 Oct 4;11(10):576. doi: 10.3390/toxins11100576. Toxins (Basel). 2019. PMID: 31590258 Free PMC article. Review.
-
A rare waterborne outbreak of Bacillus paranthracis in Shandong province, China, 2020: epidemiologic survey, genomic insights, and virulence characteristics.Emerg Microbes Infect. 2024 Dec;13(1):2348498. doi: 10.1080/22221751.2024.2348498. Epub 2024 Jun 3. Emerg Microbes Infect. 2024. PMID: 38686555 Free PMC article.
-
NMR Hydrophilic Metabolomic Analysis of Bacterial Resistance Pathways Using Multivalent Antimicrobials with Challenged and Unchallenged Wild Type and Mutated Gram-Positive Bacteria.Int J Mol Sci. 2021 Dec 19;22(24):13606. doi: 10.3390/ijms222413606. Int J Mol Sci. 2021. PMID: 34948402 Free PMC article.
References
-
- Bendich A., Machlin L. J., Scandurra O., Burton G. W., Wayner D. D. M. (1986). The antioxidant role of vitamin C. Adv. Free Radic. Biol. Med. 2 419–444. 10.1016/S8755-9668(86)80021-7 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

