Mesenchymal Stromal Cells Prevent Renal Fibrosis in a Rat Model of Unilateral Ureteral Obstruction by Suppressing the Renin-Angiotensin System via HuR
- PMID: 26866372
- PMCID: PMC4750962
- DOI: 10.1371/journal.pone.0148542
Mesenchymal Stromal Cells Prevent Renal Fibrosis in a Rat Model of Unilateral Ureteral Obstruction by Suppressing the Renin-Angiotensin System via HuR
Abstract
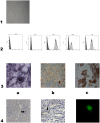
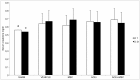
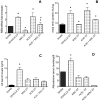
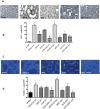
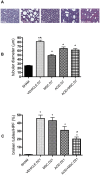
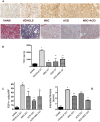
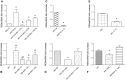
We studied Mesenchymal Stromal Cells (MSC) effects in experimental Unilateral Ureteral Obstruction (UUO), a fibrogenic renal disease. Rats were divided in 5 groups: sham, UUO, MSC treated-UUO, ACEi treated-UUO, MSC+ACEi treated- UUO. Data were collected at 1, 7, 21 days. UUO induced monocyte renal infiltration, tubular cell apoptosis, tubular atrophy, interstitial fibrosis and overexpression of TGFβ, Renin mRNA (RENmRNA), increase of Renin, Angiotensin II (AII) and aldosterone serum levels. Both lisinopril (ACEi) and MSC treatment prevented monocyte infiltration, reduced tubular cell apoptosis, renal fibrosis and TGFβ expression. Combined therapy provided a further suppression of monocyte infiltration and tubular injury. Lisinopril alone caused a rebound activation of Renin-Angiotensin System (RAS), while MSC suppressed RENmRNA and Renin synthesis and induced a decrease of AII and aldosterone serum levels. Furthermore, in in-vitro and in-vivo experiments, MSC inhibit Human antigen R (HuR) trascription, an enhancer of RENmRNA stability by IL10 release. In conclusion, we demonstrate that in UUO MSC prevent fibrosis, by decreasing HuR-dependent RENmRNA stability. Our findings give a clue to understand the molecular mechanism through which MSC may prevent fibrosis in a wide and heterogeneous number of diseases that share RAS activation as common upstream pathogenic mechanism.
Conflict of interest statement
Figures


Similar articles
-
Bone Marrow-Derived Mesenchymal Stem Cells and Their Conditioned Medium Attenuate Fibrosis in an Irreversible Model of Unilateral Ureteral Obstruction.Cell Transplant. 2015;24(12):2657-66. doi: 10.3727/096368915X687534. Epub 2015 Feb 18. Cell Transplant. 2015. PMID: 25695732
-
Human umbilical cord-derived mesenchymal stem cells conditioned medium attenuate interstitial fibrosis and stimulate the repair of tubular epithelial cells in an irreversible model of unilateral ureteral obstruction.Nephrology (Carlton). 2018 Aug;23(8):728-736. doi: 10.1111/nep.13099. Nephrology (Carlton). 2018. PMID: 28667820
-
Up-regulation of HSP47 in the mouse kidneys with unilateral ureteral obstruction.Kidney Int. 1998 Jul;54(1):110-9. doi: 10.1046/j.1523-1755.1998.00964.x. Kidney Int. 1998. PMID: 9648069
-
Biochemical-molecular markers in unilateral ureteral obstruction.Biocell. 2007;31(1):1-12. Biocell. 2007. PMID: 17665634 Review.
-
Growth factors and apoptosis in neonatal ureteral obstruction.J Am Soc Nephrol. 1996 Aug;7(8):1098-105. doi: 10.1681/ASN.V781098. J Am Soc Nephrol. 1996. PMID: 8866400 Review.
Cited by
-
The Roles of Immune Cells in the Pathogenesis of Fibrosis.Int J Mol Sci. 2020 Jul 22;21(15):5203. doi: 10.3390/ijms21155203. Int J Mol Sci. 2020. PMID: 32708044 Free PMC article. Review.
-
Safety and tolerability of autologous bone marrow mesenchymal stromal cells in ADPKD patients.Stem Cell Res Ther. 2017 May 23;8(1):116. doi: 10.1186/s13287-017-0557-7. Stem Cell Res Ther. 2017. PMID: 28535817 Free PMC article. Clinical Trial.
-
Mesenchymal stem cells protect against obstruction-induced renal fibrosis by decreasing STAT3 activation and STAT3-dependent MMP-9 production.Am J Physiol Renal Physiol. 2017 Jan 1;312(1):F25-F32. doi: 10.1152/ajprenal.00311.2016. Epub 2016 Oct 19. Am J Physiol Renal Physiol. 2017. PMID: 27760767 Free PMC article.
-
Immunotherapy of multisystem inflammatory syndrome in children (MIS-C) following COVID-19 through mesenchymal stem cells.Int Immunopharmacol. 2021 Dec;101(Pt B):108217. doi: 10.1016/j.intimp.2021.108217. Epub 2021 Oct 4. Int Immunopharmacol. 2021. PMID: 34627083 Free PMC article. Review.
-
Therapeutic potential of mesenchymal stem cells in multiple organs affected by COVID-19.Life Sci. 2021 Aug 1;278:119510. doi: 10.1016/j.lfs.2021.119510. Epub 2021 Apr 15. Life Sci. 2021. PMID: 33865879 Free PMC article. Review.
References
-
- Dal Canton A, Stanziale R, Corradi A, Andreucci VE, Migone L. Effects of acute ureteral obstruction on glomerular hemodynamics in rat kidney. Kidney Int. 1977;12: 403–11. Available: http://www.ncbi.nlm.nih.gov/pubmed/609190 - PubMed
-
- Dal Canton A, Corradi A, Stanziale R, Maruccio G, Migone L. Effects of 24-hour unilateral ureteral obstruction on glomerular hemodynamics in rat kidney. Kidney Int. 1979;15: 457–62. Available: http://www.ncbi.nlm.nih.gov/pubmed/480781 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

