A Validated Stability Indicating RP-HPLC Method for the Determination of Emtricitabine, Tenofovir Disoproxil Fumarate, Elvitegravir and Cobicistat in Pharmaceutical Dosage Form
- PMID: 26865655
- PMCID: PMC4890450
- DOI: 10.1093/chromsci/bmw004
A Validated Stability Indicating RP-HPLC Method for the Determination of Emtricitabine, Tenofovir Disoproxil Fumarate, Elvitegravir and Cobicistat in Pharmaceutical Dosage Form
Abstract
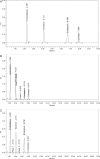
A new simple, rapid stability indicating assay method has been developed and validated for the determination of emtricitabine, tenofovir disoproxil fumarate, elvitegravir and cobicistat using reverse-phase high-performance liquid chromatography in their pharmaceutical dosage form. The chromatographic separation was performed on an ODS column (250 × 4.6 mm, 5 µm) using mobile phase A (potassium dihydrogen orthophosphate, pH adjusted to 2.5) and mobile phase B (acetonitrile) in the ratio of 55:45% v/v at a flow rate of 1 mL/min. The analytes were detected at 250 nm. The method was found to be linear in the concentration range of 2-12 µg/mL for EMT, 3-18 µg/mL for TNDF, 1.5-9 µg/mL for ELV and COB, with the coefficient value (R(2)) of >0.9990. The accuracy was measured via recovery studies and found to be acceptable, and the percentage recoveries were found in the range of 99.93-100.08 ± 0.5%. Forced degradation studies were also conducted, and the drugs were subjected to various stress conditions such as acid hydrolysis, base hydrolysis, oxidative, photolytic and thermal degradation. The proposed method was successfully validated and applied for the quantitative estimation of these drugs in both bulk and tablet dosage forms.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

Similar articles
-
UPLC-PDA Approach for Simultaneous Quantification of Elvitegravir, Tenofovir, Emtricitabine and Cobicistat in Tablets: An MS-ESI Study for Degradation Products.J Chromatogr Sci. 2024 Oct 1;62(8):719-731. doi: 10.1093/chromsci/bmad067. J Chromatogr Sci. 2024. PMID: 37691589
-
Development and validation of an LC-MS/MS assay for tenofovir and tenofovir alafenamide in human plasma and cerebrospinal fluid.J Pharm Biomed Anal. 2018 Jul 15;156:163-169. doi: 10.1016/j.jpba.2018.04.035. Epub 2018 Apr 23. J Pharm Biomed Anal. 2018. PMID: 29709783 Free PMC article.
-
Stability-Indicating HPLC Method for the Simultaneous Determination of HIV Tablet Containing Emtricitabine, Tenofovir Disoproxil Fumarate, and Rilpivirine Hydrochloride in Pharmaceutical Dosage Forms.Int Sch Res Notices. 2014 Oct 29;2014:849149. doi: 10.1155/2014/849149. eCollection 2014. Int Sch Res Notices. 2014. PMID: 27437485 Free PMC article.
-
RP-HPLC method for simultaneous estimation of bisoprolol fumarate and hydrochlorothiazide in tablet formulation.J Pharm Biomed Anal. 2010 Jul 8;52(3):362-71. doi: 10.1016/j.jpba.2009.10.021. Epub 2009 Oct 31. J Pharm Biomed Anal. 2010. PMID: 19926421
-
Development of validated stability indicating assay method for simultaneous estimation of metformin hydrochloride and vildagliptin by RP-HPLC.Drug Res (Stuttg). 2014 Mar;64(3):124-9. doi: 10.1055/s-0033-1354373. Epub 2013 Sep 19. Drug Res (Stuttg). 2014. PMID: 24081820
Cited by
-
Fabrication of a Double Core-Shell Particle-Based Magnetic Nanocomposite for Effective Adsorption-Controlled Release of Drugs.Polymers (Basel). 2022 Jun 30;14(13):2681. doi: 10.3390/polym14132681. Polymers (Basel). 2022. PMID: 35808726 Free PMC article.
References
-
- Bang L.M., Scott L.J.; Emtricitabine and antiretroviral agent for HIV infections; Drugs, (2003); 63: 2413–2424. - PubMed
-
- Saag M.S.; Emtricitabine, a new antiviral agent with activity against HIV and Hepatitis B virus; Reviews of Anti-Infective Agents, (2006); 42: 126–131. - PubMed
-
- Budawari S.; The Merck Index. 13th ed Merck and Co. Inc., Whitehouse Station, NJ, (2001); pp. 1631–1632.
-
- Martindale W.; Martindale: TheComplete DrugReference, 33rd edn. Pharmaceutical Press, London, (2002); pp. 620–642.
-
- Fung H.B., Stone E.A., Piacenti F.J.; Tenofovir disoproxil fumarate: a nucleoside reverse transcriptase inhibitor for the treatment of HIV infections; Clinical Therapeutics, (2002); 24: 1515–1548. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

