Plant ER geometry and dynamics: biophysical and cytoskeletal control during growth and biotic response
- PMID: 26862751
- PMCID: PMC5216105
- DOI: 10.1007/s00709-016-0945-3
Plant ER geometry and dynamics: biophysical and cytoskeletal control during growth and biotic response
Abstract
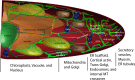
The endoplasmic reticulum (ER) is an intricate and dynamic network of membrane tubules and cisternae. In plant cells, the ER 'web' pervades the cortex and endoplasm and is continuous with adjacent cells as it passes through plasmodesmata. It is therefore the largest membranous organelle in plant cells. It performs essential functions including protein and lipid synthesis, and its morphology and movement are linked to cellular function. An emerging trend is that organelles can no longer be seen as discrete membrane-bound compartments, since they can physically interact and 'communicate' with one another. The ER may form a connecting central role in this process. This review tackles our current understanding and quantification of ER dynamics and how these change under a variety of biotic and developmental cues.
Keywords: Actin; Endoplasmic reticulum; Microtubules; Movement; Myosin.
Conflict of interest statement
Compliance with ethical standard Conflict of interest The authors declare that they have no conflict of interest.
Figures
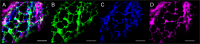
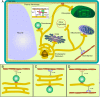
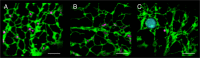
Similar articles
-
Plant cytoskeletons and the endoplasmic reticulum network organization.J Plant Physiol. 2021 Sep;264:153473. doi: 10.1016/j.jplph.2021.153473. Epub 2021 Jul 15. J Plant Physiol. 2021. PMID: 34298331 Review.
-
FrontiERs: movers and shapers of the higher plant cortical endoplasmic reticulum.Curr Opin Plant Biol. 2011 Dec;14(6):658-65. doi: 10.1016/j.pbi.2011.07.006. Epub 2011 Aug 8. Curr Opin Plant Biol. 2011. PMID: 21831697 Review.
-
Networking in the endoplasmic reticulum.Biochem Soc Trans. 2010 Jun;38(3):747-53. doi: 10.1042/BST0380747. Biochem Soc Trans. 2010. PMID: 20491660 Review.
-
The roles of membranes and associated cytoskeleton in plant virus replication and cell-to-cell movement.J Exp Bot. 2017 Dec 18;69(1):117-132. doi: 10.1093/jxb/erx334. J Exp Bot. 2017. PMID: 29036578 Review.
-
SYP73 Anchors the ER to the Actin Cytoskeleton for Maintenance of ER Integrity and Streaming in Arabidopsis.Curr Biol. 2016 Dec 5;26(23):3245-3254. doi: 10.1016/j.cub.2016.10.024. Epub 2016 Nov 17. Curr Biol. 2016. PMID: 27866894
Cited by
-
Advances in Subcellular Accumulation Design for Recombinant Protein Production in Tobacco.Biodes Res. 2024 Aug 28;6:0047. doi: 10.34133/bdr.0047. eCollection 2024. Biodes Res. 2024. PMID: 39206181 Free PMC article. Review.
-
Assembly of Dynamic P450-Mediated Metabolons-Order Versus Chaos.Curr Mol Biol Rep. 2017;3(1):37-51. doi: 10.1007/s40610-017-0053-y. Epub 2017 Feb 8. Curr Mol Biol Rep. 2017. PMID: 28255532 Free PMC article. Review.
-
Modeling Endoplasmic Reticulum Network Maintenance in a Plant Cell.Biophys J. 2017 Jul 11;113(1):214-222. doi: 10.1016/j.bpj.2017.05.046. Biophys J. 2017. PMID: 28700920 Free PMC article.
-
Ionic stress enhances ER-PM connectivity via phosphoinositide-associated SYT1 contact site expansion in Arabidopsis.Proc Natl Acad Sci U S A. 2019 Jan 22;116(4):1420-1429. doi: 10.1073/pnas.1818099116. Epub 2019 Jan 4. Proc Natl Acad Sci U S A. 2019. PMID: 30610176 Free PMC article.
-
Defining the dance: quantification and classification of endoplasmic reticulum dynamics.J Exp Bot. 2020 Mar 25;71(6):1757-1762. doi: 10.1093/jxb/erz543. J Exp Bot. 2020. PMID: 31811712 Free PMC article.
References
-
- Braun M, Wasteneys GO. Distribution and dynamics of the cytoskeleton in graviresponding protonemata and rhizoids of characean algae: exclusion of microtubules and a convergence of actin filaments in the apex suggest an actin-mediated gravitropism. Planta. 1998;205:39–50. doi: 10.1007/s004250050294. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

