Regulatory Roles of Rpl22 in Hematopoiesis: An Old Dog with New Tricks
- PMID: 26853850
- PMCID: PMC5111805
- DOI: 10.1615/critrevimmunol.v35.i5.30
Regulatory Roles of Rpl22 in Hematopoiesis: An Old Dog with New Tricks
Abstract
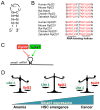
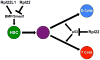
Ribosomal proteins have long been known to serve critical roles in facilitating the biogenesis of the ribosome and its ability to synthesize proteins. However, evidence is emerging that suggests ribosomal proteins are also capable of performing tissue-restricted, regulatory functions that impact normal development and pathological conditions, including cancer. The challenge in studying such regulatory functions is that elimination of many ribosomal proteins also disrupts ribosome biogenesis and/or function. Thus, it is difficult to determine whether developmental abnormalities resulting from ablation of a ribosomal protein result from loss of core ribosome functions or from loss of the regulatory function of the ribosomal protein. Rpl22, a ribosomal protein component of the large 60S subunit, provides insight into this conundrum; Rpl22 is dispensable for both ribosome biogenesis and protein synthesis yet its ablation causes tissue-restricted disruptions in development. Here we review evidence supporting the regulatory functions of Rpl22 and other ribosomal proteins.
Figures

Similar articles
-
Rpl22 Loss Selectively Impairs αβ T Cell Development by Dysregulating Endoplasmic Reticulum Stress Signaling.J Immunol. 2016 Sep 15;197(6):2280-9. doi: 10.4049/jimmunol.1600815. Epub 2016 Aug 3. J Immunol. 2016. PMID: 27489283 Free PMC article.
-
Rpl22 Loss Impairs the Development of B Lymphocytes by Activating a p53-Dependent Checkpoint.J Immunol. 2015 Jan 1;194(1):200-9. doi: 10.4049/jimmunol.1402242. J Immunol. 2015. PMID: 25416806 Free PMC article.
-
The ribosomal protein Rpl22 controls ribosome composition by directly repressing expression of its own paralog, Rpl22l1.PLoS Genet. 2013;9(8):e1003708. doi: 10.1371/journal.pgen.1003708. Epub 2013 Aug 22. PLoS Genet. 2013. PMID: 23990801 Free PMC article.
-
Plant-Specific Features of Ribosome Biogenesis.Trends Plant Sci. 2015 Nov;20(11):729-740. doi: 10.1016/j.tplants.2015.07.003. Epub 2015 Oct 11. Trends Plant Sci. 2015. PMID: 26459664 Review.
-
[About the ribosomal biogenesis in human].Med Sci (Paris). 2015 Jun-Jul;31(6-7):622-8. doi: 10.1051/medsci/20153106015. Epub 2015 Jul 7. Med Sci (Paris). 2015. PMID: 26152166 Review. French.
Cited by
-
CRISPR screening uncovers nucleolar RPL22 as a heterochromatin destabilizer and senescence driver.Nucleic Acids Res. 2024 Oct 28;52(19):11481-11499. doi: 10.1093/nar/gkae740. Nucleic Acids Res. 2024. PMID: 39258545 Free PMC article.
-
Potassium-regulated distal tubule WNK bodies are kidney-specific WNK1 dependent.Mol Biol Cell. 2018 Feb 15;29(4):499-509. doi: 10.1091/mbc.E17-08-0529. Epub 2017 Dec 13. Mol Biol Cell. 2018. PMID: 29237822 Free PMC article.
-
L22 ribosomal protein is involved in dynamin-related protein 1-mediated gastric carcinoma progression.Bioengineered. 2022 Mar;13(3):6650-6664. doi: 10.1080/21655979.2022.2045842. Bioengineered. 2022. PMID: 35230214 Free PMC article.
-
Rpl22 Loss Selectively Impairs αβ T Cell Development by Dysregulating Endoplasmic Reticulum Stress Signaling.J Immunol. 2016 Sep 15;197(6):2280-9. doi: 10.4049/jimmunol.1600815. Epub 2016 Aug 3. J Immunol. 2016. PMID: 27489283 Free PMC article.
-
Rpl22 is required for IME1 mRNA translation and meiotic induction in S. cerevisiae.Cell Div. 2016 Jul 29;11:10. doi: 10.1186/s13008-016-0024-3. eCollection 2016. Cell Div. 2016. PMID: 27478489 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

