Cannabinoid receptor 2 augments eosinophil responsiveness and aggravates allergen-induced pulmonary inflammation in mice
- PMID: 26850094
- PMCID: PMC5225803
- DOI: 10.1111/all.12858
Cannabinoid receptor 2 augments eosinophil responsiveness and aggravates allergen-induced pulmonary inflammation in mice
Abstract
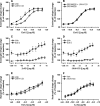
Background: Accumulation of activated eosinophils in tissue is a hallmark of allergic inflammation. The endocannabinoid 2-arachidonoylglycerol (2-AG) has been proposed to elicit eosinophil migration in a CB2 receptor/Gi/o -dependent manner. However, it has been claimed recently that this process may also involve other mechanisms such as cytokine priming and the metabolism of 2-AG into eicosanoids. Here, we explored the direct contribution of specific CB2 receptor activation to human and mouse eosinophil effector function in vitro and in vivo.
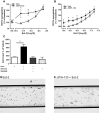
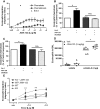
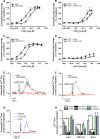
Methods: In vitro studies including CB2 expression, adhesion and migratory responsiveness, respiratory burst, degranulation, and calcium mobilization were conducted in human peripheral blood eosinophils and mouse bone marrow-derived eosinophils. Allergic airway inflammation was assessed in mouse models of acute OVA-induced asthma and directed eosinophil migration.
Results: CB2 expression was significantly higher in eosinophils from symptomatic allergic donors. The selective CB2 receptor agonist JWH-133 induced a moderate migratory response in eosinophils. However, short-term exposure to JWH-133 potently enhanced chemoattractant-induced eosinophil shape change, chemotaxis, CD11b surface expression, and adhesion as well as production of reactive oxygen species. Receptor specificity of the observed effects was confirmed in eosinophils from CB2 knockout mice and by using the selective CB2 antagonist SR144528. Of note, systemic application of JWH-133 clearly primed eosinophil-directed migration in vivo and aggravated both AHR and eosinophil influx into the airways in a CB2 -specific manner. This effect was completely absent in eosinophil-deficient ∆dblGATA mice.
Conclusion: Our data indicate that CB2 may directly contribute to the pathogenesis of eosinophil-driven diseases. Moreover, we provide new insights into the molecular mechanisms underlying the CB2 -mediated priming of eosinophils. Hence, antagonism of CB2 receptors may represent a novel pharmacological approach for the treatment of allergic inflammation and other eosinophilic disorders.
Keywords: Cannabinoid receptor 2; Eosinophils; Ovalbumin-induced asthma; Priming; airway hyperresponsiveness.
© 2016 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures


Similar articles
-
2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces the migration of EoL-1 human eosinophilic leukemia cells and human peripheral blood eosinophils.J Leukoc Biol. 2004 Nov;76(5):1002-9. doi: 10.1189/jlb.0404252. Epub 2004 Aug 17. J Leukoc Biol. 2004. PMID: 15316028
-
Cannabinoid receptor 2 as a regulator of inflammation induced oleoylethanolamide in eosinophilic asthma.J Allergy Clin Immunol. 2024 Apr;153(4):998-1009.e9. doi: 10.1016/j.jaci.2023.09.043. Epub 2023 Dec 5. J Allergy Clin Immunol. 2024. PMID: 38061443
-
Differential activation of airway eosinophils induces IL-13-mediated allergic Th2 pulmonary responses in mice.Allergy. 2015 Sep;70(9):1148-59. doi: 10.1111/all.12655. Epub 2015 Jun 14. Allergy. 2015. PMID: 26009788 Free PMC article.
-
Airway hyperresponsiveness: first eosinophils and then neuropeptides.Int J Immunopharmacol. 1997 Sep-Oct;19(9-10):517-27. doi: 10.1016/s0192-0561(97)00085-4. Int J Immunopharmacol. 1997. PMID: 9637348 Review.
-
The Role of MIF on Eosinophil Biology and Eosinophilic Inflammation.Clin Rev Allergy Immunol. 2020 Feb;58(1):15-24. doi: 10.1007/s12016-019-08726-z. Clin Rev Allergy Immunol. 2020. PMID: 30680604 Review.
Cited by
-
Apolipoprotein A-IV acts as an endogenous anti-inflammatory protein and is reduced in treatment-naïve allergic patients and allergen-challenged mice.Allergy. 2020 Feb;75(2):392-402. doi: 10.1111/all.14022. Epub 2019 Sep 10. Allergy. 2020. PMID: 31408538 Free PMC article.
-
Regulation of Eosinophil and Group 2 Innate Lymphoid Cell Trafficking in Asthma.Front Med (Lausanne). 2017 Aug 11;4:136. doi: 10.3389/fmed.2017.00136. eCollection 2017. Front Med (Lausanne). 2017. PMID: 28848734 Free PMC article.
-
Emerging Role of Phospholipase-Derived Cleavage Products in Regulating Eosinophil Activity: Focus on Lysophospholipids, Polyunsaturated Fatty Acids and Eicosanoids.Int J Mol Sci. 2021 Apr 21;22(9):4356. doi: 10.3390/ijms22094356. Int J Mol Sci. 2021. PMID: 33919453 Free PMC article. Review.
-
The Interplay between the Immune and the Endocannabinoid Systems in Cancer.Cells. 2021 May 21;10(6):1282. doi: 10.3390/cells10061282. Cells. 2021. PMID: 34064197 Free PMC article. Review.
-
Peroxisome Proliferator-Activated Receptors as a Therapeutic Target in Asthma.PPAR Res. 2020 Jan 14;2020:8906968. doi: 10.1155/2020/8906968. eCollection 2020. PPAR Res. 2020. PMID: 32395125 Free PMC article. Review.
References
-
- Straumann A, Simon H‐U. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol 2005;115:418–419. - PubMed
-
- Wedemeyer J, Vosskuhl K. Role of gastrointestinal eosinophils in inflammatory bowel disease and intestinal tumours. Best Pract Res Clin Gastroenterol 2008;22:537–549. - PubMed
-
- Simon D, Simon H‐U. Eosinophilic disorders. J Allergy Clin Immunol 2007;119:1291–1300. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

