Generating Peripheral Blood Derived Lymphocytes Reacting Against Autologous Primary AML Blasts
- PMID: 26849076
- PMCID: PMC4746019
- DOI: 10.1097/CJI.0000000000000107
Generating Peripheral Blood Derived Lymphocytes Reacting Against Autologous Primary AML Blasts
Abstract
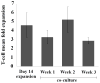
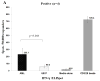
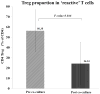
Expanding on our prior studies with cord blood T cells, we hypothesized that primary acute myeloid leukemia (AML)-reactive autologous T cells could be generated ex vivo under immunomodulatory conditions. We purified AML and T cells from 8 newly diagnosed high-risk patients. After 2 weeks expansion, T cells were stimulated with interferon-γ-treated autologous AML weekly × 3, interleukin-15, and agonistic anti-CD28 antibody. Cytotoxic T cells and ELISpot assays tested functionality; reverse transcriptase quantitative polymerase chain reaction tested AML and T-cell gene expression profiles. On the basis of combined positive ELIspot and cytotoxic T cells assays, T cells reactive against AML were generated in 5 of 8 patients. Treg proportion declined after cocultures in reactive T-cell samples. AML-reactive T cells displayed an activated gene expression profile. "Resistant" AML blasts displayed genes associated with immunosuppressive myeloid-derived suppressor cells. We discuss our approach to creating primary AML-reactive autologous T cell and limitations that require further work. Our study provides a platform for future research targeting on generating autologous leukemia-reactive T cells.
Conflict of interest statement
Figures


Similar articles
-
Generation of T-cell lines to autologous acute myeloid leukemia cells by competitive limiting dilution culture of acute myeloid leukemia mononuclear cells.Exp Hematol. 2008 Apr;36(4):486-94. doi: 10.1016/j.exphem.2007.11.012. Epub 2008 Jan 30. Exp Hematol. 2008. PMID: 18249062
-
Immunotherapy for patients with acute myeloid leukemia using autologous dendritic cells generated from leukemic blasts.Int J Oncol. 2006 Apr;28(4):855-61. Int J Oncol. 2006. PMID: 16525634 Clinical Trial.
-
CTLA-4 blockade by a human MAb enhances the capacity of AML-derived DC to induce T-cell responses against AML cells in an autologous culture system.Cytotherapy. 2006;8(1):3-12. doi: 10.1080/14653240500499507. Cytotherapy. 2006. PMID: 16627340
-
Antileukemic T-cell responses can be predicted by the composition of specific regulatory T-cell subpopulations.J Immunother. 2013 May;36(4):223-37. doi: 10.1097/CJI.0b013e31829180e7. J Immunother. 2013. PMID: 23603857
-
Clinical grade expansion of CD45RA, CD45RO, and CD62L-positive T-cell lines from HLA-compatible donors: high cytotoxic potential against AML and ALL cells.Exp Hematol. 2006 Apr;34(4):475-85. doi: 10.1016/j.exphem.2005.12.012. Exp Hematol. 2006. PMID: 16569594
Cited by
-
The characteristics of circRNA as competing endogenous RNA in pathogenesis of acute myeloid leukemia.BMC Cancer. 2021 Mar 15;21(1):277. doi: 10.1186/s12885-021-08029-7. BMC Cancer. 2021. PMID: 33722210 Free PMC article.
-
Cancer immune therapy for myeloid malignancies: present and future.Semin Immunopathol. 2019 Jan;41(1):97-109. doi: 10.1007/s00281-018-0693-x. Epub 2018 Jul 9. Semin Immunopathol. 2019. PMID: 29987478 Review.
-
Characterization and dynamics of specific T cells against nucleophosmin-1 (NPM1)-mutated peptides in patients with NPM1-mutated acute myeloid leukemia.Oncotarget. 2019 Jan 25;10(8):869-882. doi: 10.18632/oncotarget.26617. eCollection 2019 Jan 25. Oncotarget. 2019. PMID: 30783516 Free PMC article.
-
A novel immune prognostic model of non-M3 acute myeloid leukemia.Am J Transl Res. 2022 Aug 15;14(8):5308-5325. eCollection 2022. Am J Transl Res. 2022. PMID: 36105048 Free PMC article.
-
Neoantigen-Specific T-Cell Immune Responses: The Paradigm of NPM1-Mutated Acute Myeloid Leukemia.Int J Mol Sci. 2021 Aug 25;22(17):9159. doi: 10.3390/ijms22179159. Int J Mol Sci. 2021. PMID: 34502069 Free PMC article. Review.
References
-
- Amrolia PJ, Reid SD, Gao L, et al. Allorestricted cytotoxic T cells specific for human CD45 show potent antileukemic activity. Blood. 2003;101(3):1007–1014. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

