Zoledronate blocks geranylgeranylation not farnesylation to suppress human osteosarcoma U2OS cells metastasis by EMT via Rho A activation and FAK-inhibited JNK and p38 pathways
- PMID: 26848867
- PMCID: PMC4891081
- DOI: 10.18632/oncotarget.7138
Zoledronate blocks geranylgeranylation not farnesylation to suppress human osteosarcoma U2OS cells metastasis by EMT via Rho A activation and FAK-inhibited JNK and p38 pathways
Abstract
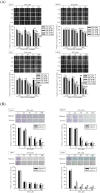
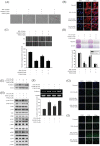
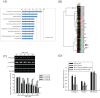
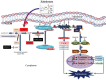
Zoledronate is a standard treatment for preventing skeletal complications of osteoporosis and some types of cancer associated with bone metastases, but we little know whether the effect of zoledronate on metastasis of osteosarcoma. Here, we investigated the inhibitory effects of zoledronate on cell viability, motility, migration and invasion of 4 osteosarcoma cell lines (Saos2, MG-63, HOS and U2OS) by affecting cell morphology, epithelial-mesenchymal transition (EMT) and cytoskeletal organization as well as induction of E-cadherin and reduction of N-cadherin with activation of transcription factors Slug and Twist, especially in U2OS cells. Zoledronate decreased JNK and p38 phosphorylation and upper streams of focal adhesion kinase (FAK) and Src to suppress the motility, invasiveness and migration of U2OS cells. In addition to zoledronate-inhibited Rho A and Cdc42 membrane translocation and GTPγS activities, the anti-metastatic effects in U2OS cells including inhibition of adhesion were reversed by geranylgeraniol, but not farnesol. In conclusion, Zoledronate blocks geranylgeranylation not farnesylation to suppress human osteosarcoma U2OS cell-matrix and cell-cell interactions, migration potential, the invasive activity, and the adhesive ability by EMT via Rho A activation and FAK-inhibited JNK and p38 pathways.
Keywords: Rho A; U2OS; geranylgeranylation; metastasis; zoledronate.
Conflict of interest statement
The authors declare that no conflicts of interest exist.
Figures



Similar articles
-
Zoledronate inhibits endothelial cell adhesion, migration and survival through the suppression of multiple, prenylation-dependent signaling pathways.J Thromb Haemost. 2007 Jan;5(1):166-73. doi: 10.1111/j.1538-7836.2006.02259.x. Epub 2006 Oct 13. J Thromb Haemost. 2007. PMID: 17059425
-
3-Hydroxyflavone inhibits human osteosarcoma U2OS and 143B cells metastasis by affecting EMT and repressing u-PA/MMP-2 via FAK-Src to MEK/ERK and RhoA/MLC2 pathways and reduces 143B tumor growth in vivo.Food Chem Toxicol. 2016 Nov;97:177-186. doi: 10.1016/j.fct.2016.09.006. Epub 2016 Sep 4. Food Chem Toxicol. 2016. PMID: 27600294
-
Nitidine chloride suppresses epithelial-to-mesenchymal transition in osteosarcoma cell migration and invasion through Akt/GSK-3β/Snail signaling pathway.Oncol Rep. 2016 Aug;36(2):1023-9. doi: 10.3892/or.2016.4846. Epub 2016 Jun 2. Oncol Rep. 2016. PMID: 27279040
-
AKT-ions with a TWIST between EMT and MET.Oncotarget. 2016 Sep 20;7(38):62767-62777. doi: 10.18632/oncotarget.11232. Oncotarget. 2016. PMID: 27623213 Free PMC article. Review.
-
New insights into molecular and cellular mechanisms of zoledronate in human osteosarcoma.Pharmacol Ther. 2020 Oct;214:107611. doi: 10.1016/j.pharmthera.2020.107611. Epub 2020 Jun 18. Pharmacol Ther. 2020. PMID: 32565177 Review.
Cited by
-
Cucurbitacin E inhibits osteosarcoma cells proliferation and invasion through attenuation of PI3K/AKT/mTOR signalling pathway.Biosci Rep. 2016 Nov 8;36(6):e00405. doi: 10.1042/BSR20160165. Print 2016 Dec. Biosci Rep. 2016. Retraction in: Biosci Rep. 2024 Feb 28;44(2):BSR-2016-0165_RET. doi: 10.1042/BSR-2016-0165_RET PMID: 27653525 Free PMC article. Retracted.
-
The role of programmed cell death in osteosarcoma: From pathogenesis to therapy.Cancer Med. 2024 May;13(10):e7303. doi: 10.1002/cam4.7303. Cancer Med. 2024. PMID: 38800967 Free PMC article. Review.
-
Expression and role of microRNA-1271 in the pathogenesis of osteosarcoma.Exp Ther Med. 2018 Feb;15(2):1934-1940. doi: 10.3892/etm.2017.5649. Epub 2017 Dec 15. Exp Ther Med. 2018. PMID: 29434787 Free PMC article.
-
MMP-11 promoted the oral cancer migration and Fak/Src activation.Oncotarget. 2017 May 16;8(20):32783-32793. doi: 10.18632/oncotarget.15824. Oncotarget. 2017. PMID: 28427180 Free PMC article.
-
Ping-Chong-Jiang-Ni Formula Induces Apoptosis and Inhibits Proliferation of Human Ectopic Endometrial Stromal Cells in Endometriosis via the Activation of JNK Signaling Pathway.Evid Based Complement Alternat Med. 2017;2017:6489427. doi: 10.1155/2017/6489427. Epub 2017 Jun 1. Evid Based Complement Alternat Med. 2017. PMID: 28656053 Free PMC article.
References
-
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer treatment and research. 2009;152:3–13. - PubMed
-
- Oertel S, Blattmann C, Rieken S, Jensen A, Combs SE, Huber PE, Bischof M, Kulozik A, Debus J, Schulz-Ertner D. Radiotherapy in the treatment of primary osteosarcoma—a single center experience. Tumori. 2010;96:582–588. - PubMed
-
- Hsieh YS, Chu SC, Yang SF, Chen PN, Liu YC, Lu KH. Silibinin suppresses human osteosarcoma MG-63 cell invasion by inhibiting the ERK-dependent c-Jun/AP-1 induction of MMP-2. Carcinogenesis. 2007;28:977–987. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

