IL15 Trispecific Killer Engagers (TriKE) Make Natural Killer Cells Specific to CD33+ Targets While Also Inducing Persistence, In Vivo Expansion, and Enhanced Function
- PMID: 26847056
- PMCID: PMC4947440
- DOI: 10.1158/1078-0432.CCR-15-2710
IL15 Trispecific Killer Engagers (TriKE) Make Natural Killer Cells Specific to CD33+ Targets While Also Inducing Persistence, In Vivo Expansion, and Enhanced Function
Abstract
Purpose: The effectiveness of NK cell infusions to induce leukemic remission is limited by lack of both antigen specificity and in vivo expansion. To address the first issue, we previously generated a bispecific killer engager (BiKE) containing single-chain scFv against CD16 and CD33 to create an immunologic synapse between NK cells and CD33(+) myeloid targets. We have now incorporated a novel modified human IL15 crosslinker, producing a 161533 trispecific killer engager (TriKE) to induce expansion, priming, and survival, which we hypothesize will enhance clinical efficacy.
Experimental design: Reagents were tested in proliferation and functional assays and in an in vivo xenograft model of AML.
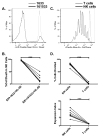
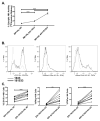
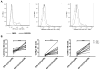
Results: When compared with the 1633 BiKE, the 161533 TriKE induced superior NK cell cytotoxicity, degranulation, and cytokine production against CD33(+) HL-60 targets and increased NK survival and proliferation. Specificity was shown by the ability of a 1615EpCAM TriKE to kill CD33-EpCAM(+) targets. Using NK cells from patients after allogeneic stem cell transplantation when NK cell function is defective, the 161533 TriKE restored potent NK function against primary AML targets and induced specific NK cell proliferation. These results were confirmed in an immunodeficient mouse HL-60-Luc tumor model where the 161533 TriKE exhibited superior antitumor activity and induced in vivo persistence and survival of human NK cells for at least 3 weeks.
Conclusions: Off-the-shelf 161533 TriKE imparts antigen specificity and promotes in vivo persistence, activation, and survival of NK cells. These qualities are ideal for NK cell therapy of myeloid malignancies or targeting antigens of solid tumors. Clin Cancer Res; 22(14); 3440-50. ©2016 AACRSee related commentary by Talmadge, p. 3419.
©2016 American Association for Cancer Research.
Conflict of interest statement
Conflict-of-interest disclosure: Drs. Vallera and Miller are members of the Oxis Biotech Scientific Advisory Board and hold equity in the company. This relationship has been reviewed and managed by the University of Minnesota in accordance with its conflict of interest policies.
Figures



Comment in
-
Genetically Engineered Multivalent Proteins for Targeted Immunotherapy.Clin Cancer Res. 2016 Jul 15;22(14):3419-21. doi: 10.1158/1078-0432.CCR-16-0246. Epub 2016 Mar 23. Clin Cancer Res. 2016. PMID: 27009744 Free PMC article.
-
TriKEs and BiKEs join CARs on the cancer immunotherapy highway.Hum Vaccin Immunother. 2016 Nov;12(11):2790-2796. doi: 10.1080/21645515.2016.1198455. Epub 2016 Jun 20. Hum Vaccin Immunother. 2016. PMID: 27322989 Free PMC article.
Similar articles
-
Enhanced ADCC and NK Cell Activation of an Anticarcinoma Bispecific Antibody by Genetic Insertion of a Modified IL-15 Cross-linker.Mol Ther. 2016 Aug;24(7):1312-22. doi: 10.1038/mt.2016.88. Epub 2016 May 9. Mol Ther. 2016. PMID: 27157665 Free PMC article.
-
Potent Cytolytic Activity and Specific IL15 Delivery in a Second-Generation Trispecific Killer Engager.Cancer Immunol Res. 2020 Sep;8(9):1139-1149. doi: 10.1158/2326-6066.CIR-19-0837. Epub 2020 Jul 13. Cancer Immunol Res. 2020. PMID: 32661096 Free PMC article.
-
Engineering of Anti-CD133 Trispecific Molecule Capable of Inducing NK Expansion and Driving Antibody-Dependent Cell-Mediated Cytotoxicity.Cancer Res Treat. 2017 Oct;49(4):1140-1152. doi: 10.4143/crt.2016.491. Epub 2017 Feb 20. Cancer Res Treat. 2017. PMID: 28231426 Free PMC article.
-
Therapeutic applications: natural killer cells in the clinic.Hematology Am Soc Hematol Educ Program. 2013;2013:247-53. doi: 10.1182/asheducation-2013.1.247. Hematology Am Soc Hematol Educ Program. 2013. PMID: 24319187 Review.
-
Natural killer cells unleashed: Checkpoint receptor blockade and BiKE/TriKE utilization in NK-mediated anti-tumor immunotherapy.Semin Immunol. 2017 Jun;31:64-75. doi: 10.1016/j.smim.2017.07.011. Epub 2017 Sep 5. Semin Immunol. 2017. PMID: 28882429 Free PMC article. Review.
Cited by
-
Bispecific antibodies in cancer immunotherapy.Hum Vaccin Immunother. 2016 Oct 2;12(10):2491-2500. doi: 10.1080/21645515.2016.1187802. Epub 2016 Jun 1. Hum Vaccin Immunother. 2016. PMID: 27249163 Free PMC article. Review.
-
Heterodimeric IL-15 in Cancer Immunotherapy.Cancers (Basel). 2021 Feb 17;13(4):837. doi: 10.3390/cancers13040837. Cancers (Basel). 2021. PMID: 33671252 Free PMC article. Review.
-
Antibody therapies for the treatment of acute myeloid leukemia: exploring current and emerging therapeutic targets.Expert Opin Investig Drugs. 2023 Feb;32(2):107-125. doi: 10.1080/13543784.2023.2179482. Epub 2023 Feb 26. Expert Opin Investig Drugs. 2023. PMID: 36762937 Free PMC article. Review.
-
Natural Killer Cells in Myeloid Malignancies: Immune Surveillance, NK Cell Dysfunction, and Pharmacological Opportunities to Bolster the Endogenous NK Cells.Front Immunol. 2019 Oct 11;10:2357. doi: 10.3389/fimmu.2019.02357. eCollection 2019. Front Immunol. 2019. PMID: 31681270 Free PMC article. Review.
-
T-BET and EOMES Accelerate and Enhance Functional Differentiation of Human Natural Killer Cells.Front Immunol. 2021 Sep 24;12:732511. doi: 10.3389/fimmu.2021.732511. eCollection 2021. Front Immunol. 2021. PMID: 34630413 Free PMC article.
References
-
- Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. - PubMed
-
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. - PubMed
-
- Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lewis D, Hippen K, McGlave P, Weisdorf DJ, Blazar BR, Miller JS. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123:3855–63. - PMC - PubMed
-
- Bell CJ, Sun Y, Nowak UM, Clark J, Howlett S, Pekalski ML, Yang X, Ast O, Waldhauer I, Freimoser-Grundschober A, Moessner E, Umana P, Klein C, Hosse RJ, Wicker LS, Peterson LB. Sustained in vivo signaling by long-lived IL-2 induces prolonged increases of regulatory T cells. J Autoimmun. 2015;56:66–80. - PMC - PubMed
-
- Lanier LL, Ruitenberg JJ, Phillips JH. Functional and biochemical analysis of CD16 antigen on natural killer cells and granulocytes. J Immunol. 1988;141:3478–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

