Thioredoxin 1 protects astrocytes from oxidative stress by maintaining peroxiredoxin activity
- PMID: 26846911
- PMCID: PMC4768962
- DOI: 10.3892/mmr.2016.4855
Thioredoxin 1 protects astrocytes from oxidative stress by maintaining peroxiredoxin activity
Abstract
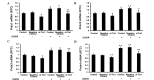
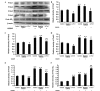
Previous studies have demonstrated that thioredoxin 1 (Trx1) exerts neuroprotective effects against cerebral ischemia/reperfusion injury caused by oxidative stress. While Trx1 is known to maintain the anti‑oxidant activity of 2‑Cys peroxiredoxins (Prdxs), the underlying mechanisms of its protective effects have remained to be elucidated, which was the aim of the present study. For this, an in vitro ischemic model of hypoxemia lasting for 4 h, followed by 24 h of reperfusion was used. Primary astrocytes from neonatal rats were pre‑treated with small interfering RNA targeting Trx1 prior to oxygen glucose deprivation/reperfusion (OGD/R). MTS and lactate dehydrogenase assays were performed to evaluate cell viability. Reverse transcription‑quantitative polymerase chain reaction (RT‑qPCR) and western blot analysis were employed to assess the mRNA and protein expression levels of Prdx1‑4 and Prdx‑SO3. Furthermore, a dual luciferase reporter assay was used to assess the interaction between activator protein‑1 (AP‑1) and Trx1. The present study demonstrated that OGD/R decreased the cell viability and increased cellular damage, which was more marked following Trx1 knockdown. The expression of Prdx1‑4 and Prdx‑SO3 protein was higher in the cells subjected to OGD/R. Knockdown of Trx1 markedly decreased the levels of Prdx1‑4 but increased Prdx‑SO3 mRNA and protein levels. The results of the present study also suggested that AP‑1 directly activated the expression of Trx1. The present study demonstrated that Trx1 exerts its neuroprotective effects by preventing oxidative stress in astrocytes via maintaining Prdx expression.
Figures
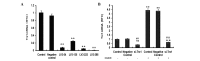
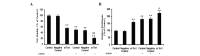
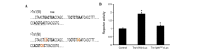
Similar articles
-
Silencing thioredoxin1 exacerbates damage of astrocytes exposed to OGD/R by aggravating apoptosis through the Actin-Ras2-cAMP-PKA pathway.Int J Neurosci. 2018 Jun;128(6):512-519. doi: 10.1080/00207454.2017.1398159. Epub 2017 Nov 16. Int J Neurosci. 2018. PMID: 29073813
-
Neuroprotective effects of sulfiredoxin-1 during cerebral ischemia/reperfusion oxidative stress injury in rats.Brain Res Bull. 2017 Jun;132:99-108. doi: 10.1016/j.brainresbull.2017.05.012. Epub 2017 May 24. Brain Res Bull. 2017. PMID: 28552673
-
Sulfiredoxin-1 exerts anti-apoptotic and neuroprotective effects against oxidative stress-induced injury in rat cortical astrocytes following exposure to oxygen-glucose deprivation and hydrogen peroxide.Int J Mol Med. 2015 Jul;36(1):43-52. doi: 10.3892/ijmm.2015.2205. Epub 2015 May 8. Int J Mol Med. 2015. PMID: 25955519 Free PMC article.
-
The Functions of Thioredoxin 1 in Neurodegeneration.Antioxid Redox Signal. 2022 May;36(13-15):1023-1036. doi: 10.1089/ars.2021.0186. Epub 2021 Dec 31. Antioxid Redox Signal. 2022. PMID: 34465198 Review.
-
Thioredoxin regulation of ischemic preconditioning.Antioxid Redox Signal. 2004 Apr;6(2):405-12. doi: 10.1089/152308604322899477. Antioxid Redox Signal. 2004. PMID: 15025942 Review.
Cited by
-
Peroxiredoxin 1 alleviates oxygen-glucose deprivation/ reoxygenation injury in N2a cells via suppressing the JNK/caspase-3 pathway.Iran J Basic Med Sci. 2023;26(11):1305-1312. doi: 10.22038/IJBMS.2023.71390.15528. Iran J Basic Med Sci. 2023. PMID: 37886002 Free PMC article.
-
Cysteine, Glutathione, and Thiol Redox Balance in Astrocytes.Antioxidants (Basel). 2017 Aug 3;6(3):62. doi: 10.3390/antiox6030062. Antioxidants (Basel). 2017. PMID: 28771170 Free PMC article. Review.
-
Protective Role of Transduced Tat-Thioredoxin1 (Trx1) against Oxidative Stress-Induced Neuronal Cell Death via ASK1-MAPK Signal Pathway.Biomol Ther (Seoul). 2021 May 1;29(3):321-330. doi: 10.4062/biomolther.2020.154. Biomol Ther (Seoul). 2021. PMID: 33436533 Free PMC article.
-
Role and underlying mechanism of SPATA12 in oxidative damage.Oncol Lett. 2018 Mar;15(3):3676-3684. doi: 10.3892/ol.2018.7749. Epub 2018 Jan 8. Oncol Lett. 2018. PMID: 29467887 Free PMC article.
-
In Vitro Modulation of Redox and Metabolism Interplay at the Brain Vascular Endothelium: Genomic and Proteomic Profiles of Sulforaphane Activity.Sci Rep. 2018 Aug 23;8(1):12708. doi: 10.1038/s41598-018-31137-7. Sci Rep. 2018. PMID: 30139948 Free PMC article.
References
-
- Calabrese V, Cornelius C, Mancuso C, Pennisi G, Calafato S, Bellia F, Bates TE, Giuffrida Stella AM, Schapira T, Dinkova Kostova AT, Rizzarelli E. Cellular stress response: A novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res. 2008;33:2444–2471. doi: 10.1007/s11064-008-9775-9. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

