Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan
- PMID: 26840489
- PMCID: PMC4845101
- DOI: 10.1038/nature16932
Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan
Abstract
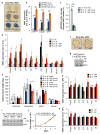
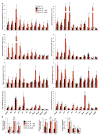
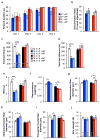
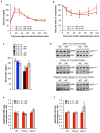
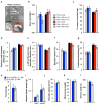
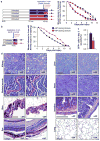
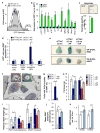
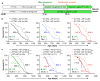
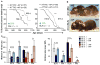
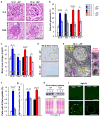
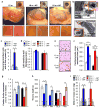
Cellular senescence, a stress-induced irreversible growth arrest often characterized by expression of p16(Ink4a) (encoded by the Ink4a/Arf locus, also known as Cdkn2a) and a distinctive secretory phenotype, prevents the proliferation of preneoplastic cells and has beneficial roles in tissue remodelling during embryogenesis and wound healing. Senescent cells accumulate in various tissues and organs over time, and have been speculated to have a role in ageing. To explore the physiological relevance and consequences of naturally occurring senescent cells, here we use a previously established transgene, INK-ATTAC, to induce apoptosis in p16(Ink4a)-expressing cells of wild-type mice by injection of AP20187 twice a week starting at one year of age. We show that compared to vehicle alone, AP20187 treatment extended median lifespan in both male and female mice of two distinct genetic backgrounds. The clearance of p16(Ink4a)-positive cells delayed tumorigenesis and attenuated age-related deterioration of several organs without apparent side effects, including kidney, heart and fat, where clearance preserved the functionality of glomeruli, cardio-protective KATP channels and adipocytes, respectively. Thus, p16(Ink4a)-positive cells that accumulate during adulthood negatively influence lifespan and promote age-dependent changes in several organs, and their therapeutic removal may be an attractive approach to extend healthy lifespan.
Figures




Comment in
-
Ageing: Out with the old.Nature. 2016 Feb 11;530(7589):164-5. doi: 10.1038/nature16875. Epub 2016 Feb 3. Nature. 2016. PMID: 26840486 Free PMC article. No abstract available.
-
Ageing: Anti-ageing formula.Nat Rev Mol Cell Biol. 2016 Mar;17(3):134-5. doi: 10.1038/nrm.2016.16. Epub 2016 Feb 17. Nat Rev Mol Cell Biol. 2016. PMID: 26883004 No abstract available.
Similar articles
-
Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders.Nature. 2011 Nov 2;479(7372):232-6. doi: 10.1038/nature10600. Nature. 2011. PMID: 22048312 Free PMC article.
-
Progressive Cellular Senescence Mediates Renal Dysfunction in Ischemic Nephropathy.J Am Soc Nephrol. 2021 Aug;32(8):1987-2004. doi: 10.1681/ASN.2020091373. Epub 2021 Jun 16. J Am Soc Nephrol. 2021. PMID: 34135081 Free PMC article.
-
Aging of mice is associated with p16(Ink4a)- and β-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells.Aging (Albany NY). 2016 Jul;8(7):1294-315. doi: 10.18632/aging.100991. Aging (Albany NY). 2016. PMID: 27391570 Free PMC article.
-
Cellular Senescence in Diabetes Mellitus: Distinct Senotherapeutic Strategies for Adipose Tissue and Pancreatic β Cells.Front Endocrinol (Lausanne). 2022 Mar 31;13:869414. doi: 10.3389/fendo.2022.869414. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35432205 Free PMC article. Review.
-
Involvement of the INK4a/Arf gene locus in senescence.Aging Cell. 2003 Jun;2(3):145-50. doi: 10.1046/j.1474-9728.2003.00048.x. Aging Cell. 2003. PMID: 12882406 Review.
Cited by
-
Targeting immunosenescence for improved tumor immunotherapy.MedComm (2020). 2024 Oct 28;5(11):e777. doi: 10.1002/mco2.777. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39473905 Free PMC article. Review.
-
Immune therapeutic strategies for the senescent tumor microenvironment.Br J Cancer. 2024 Oct 28. doi: 10.1038/s41416-024-02865-7. Online ahead of print. Br J Cancer. 2024. PMID: 39468331 Review.
-
Partial reduction of interleukin-33 signaling improves senescence and renal injury in diabetic nephropathy.MedComm (2020). 2024 Oct 24;5(11):e742. doi: 10.1002/mco2.742. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39465143 Free PMC article.
-
Identification of lipid senolytics targeting senescent cells through ferroptosis induction.bioRxiv [Preprint]. 2024 Oct 14:2024.10.14.618023. doi: 10.1101/2024.10.14.618023. bioRxiv. 2024. PMID: 39463954 Free PMC article. Preprint.
-
Mitochondrial fatty acid oxidation drives senescence.Sci Adv. 2024 Oct 25;10(43):eado5887. doi: 10.1126/sciadv.ado5887. Epub 2024 Oct 25. Sci Adv. 2024. PMID: 39454000 Free PMC article.
References
-
- Sharpless NE, Sherr CJ. Forging a signature of in vivo senescence. Nat Rev Cancer. 2015;15:397–408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

