Effects of Acyclovir, Foscarnet, and Ribonucleotides on Herpes Simplex Virus-1 DNA Polymerase: Mechanistic Insights and a Novel Mechanism for Preventing Stable Incorporation of Ribonucleotides into DNA
- PMID: 26836009
- PMCID: PMC5551684
- DOI: 10.1021/acs.biochem.6b00065
Effects of Acyclovir, Foscarnet, and Ribonucleotides on Herpes Simplex Virus-1 DNA Polymerase: Mechanistic Insights and a Novel Mechanism for Preventing Stable Incorporation of Ribonucleotides into DNA
Abstract
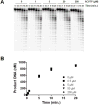
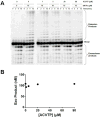
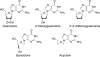
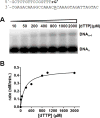
We examined the impact of two clinically approved anti-herpes drugs, acyclovir and Forscarnet (phosphonoformate), on the exonuclease activity of the herpes simplex virus-1 DNA polymerase, UL30. Acyclovir triphosphate and Foscarnet, along with the closely related phosphonoacetic acid, did not affect exonuclease activity on single-stranded DNA. Furthermore, blocking the polymerase active site due to either binding of Foscarnet or phosphonoacetic acid to the E-DNA complex or polymerization of acyclovir onto the DNA also had a minimal effect on exonuclease activity. The inability of the exonuclease to excise acyclovir from the primer 3'-terminus results from the altered sugar structure directly impeding phosphodiester bond hydrolysis as opposed to inhibiting binding, unwinding of the DNA by the exonuclease, or transfer of the DNA from the polymerase to the exonuclease. Removing the 3'-hydroxyl or the 2'-carbon from the nucleotide at the 3'-terminus of the primer strongly inhibited exonuclease activity, although addition of a 2'-hydroxyl did not affect exonuclease activity. The biological consequences of these results are twofold. First, the ability of acyclovir and Foscarnet to block dNTP polymerization without impacting exonuclease activity raises the possibility that their effects on herpes replication may involve both direct inhibition of dNTP polymerization and exonuclease-mediated destruction of herpes DNA. Second, the ability of the exonuclease to rapidly remove a ribonucleotide at the primer 3'-terminus in combination with the polymerase not efficiently adding dNTPs onto this primer provides a novel mechanism by which the herpes replication machinery can prevent incorporation of ribonucleotides into newly synthesized DNA.
Figures
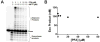


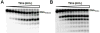

Similar articles
-
DNA polymerase mutations in drug-resistant herpes simplex virus mutants determine in vivo neurovirulence and drug-enzyme interactions.Antivir Ther. 2007;12(5):719-32. Antivir Ther. 2007. PMID: 17713155
-
Differential impact of various substitutions at codon 715 in region II of HSV-1 and HCMV DNA polymerases.Antiviral Res. 2021 Apr;188:105046. doi: 10.1016/j.antiviral.2021.105046. Epub 2021 Feb 12. Antiviral Res. 2021. PMID: 33588012
-
The Anti-Human Immunodeficiency Virus Drug Tenofovir, a Reverse Transcriptase Inhibitor, Also Targets the Herpes Simplex Virus DNA Polymerase.J Infect Dis. 2018 Feb 14;217(5):790-801. doi: 10.1093/infdis/jix605. J Infect Dis. 2018. PMID: 29186456
-
Distribution and effects of amino acid changes in drug-resistant α and β herpesviruses DNA polymerase.Nucleic Acids Res. 2016 Nov 16;44(20):9530-9554. doi: 10.1093/nar/gkw875. Epub 2016 Sep 29. Nucleic Acids Res. 2016. PMID: 27694307 Free PMC article. Review.
-
[Resistance of herpes simplex viruses to antiviral drugs].Pathol Biol (Paris). 1993 Feb;41(2):172-7. Pathol Biol (Paris). 1993. PMID: 8392160 Review. French.
Cited by
-
Different Divalent Cations Alter the Kinetics and Fidelity of DNA Polymerases.J Biol Chem. 2016 Sep 30;291(40):20869-20875. doi: 10.1074/jbc.R116.742494. Epub 2016 Jul 26. J Biol Chem. 2016. PMID: 27462081 Free PMC article. Review.
-
Evaluation of the effect of hydro alcoholic extract of cinnamon on herpes simplex virus-1.Dent Res J (Isfahan). 2020 Mar 17;17(2):114-119. eCollection 2020 Mar-Apr. Dent Res J (Isfahan). 2020. PMID: 32435433 Free PMC article.
-
Guanine α-carboxy nucleoside phosphonate (G-α-CNP) shows a different inhibitory kinetic profile against the DNA polymerases of human immunodeficiency virus (HIV) and herpes viruses.Biochem Pharmacol. 2017 Jul 15;136:51-61. doi: 10.1016/j.bcp.2017.04.001. Epub 2017 Apr 6. Biochem Pharmacol. 2017. PMID: 28390939 Free PMC article.
-
Novel Antibiotics Targeting Bacterial Replicative DNA Polymerases.Antibiotics (Basel). 2020 Nov 4;9(11):776. doi: 10.3390/antibiotics9110776. Antibiotics (Basel). 2020. PMID: 33158178 Free PMC article. Review.
-
Loss of DNA methylation in zebrafish embryos activates retrotransposons to trigger antiviral signaling.Development. 2017 Aug 15;144(16):2925-2939. doi: 10.1242/dev.147629. Epub 2017 Jul 11. Development. 2017. PMID: 28698226 Free PMC article.
References
-
- Crumpacker CS, Schnipper LE, Chartrand P, Knopf KW. Genetic mechanisms of resistance to acyclovir in herpes simplex virus. Am J Med. 1982;73:361–368. - PubMed
-
- Crumpacker CS, Chartrand P, Subak-Sharpe JH, Wilkie NM. Resistance of herpes simplex virus to acycloguanosine–genetic and physical analysis. Virology. 1980;105:171–184. - PubMed
-
- Coen DM, Schaffer PA, Furman PA, Keller PM, St Clair MH. Biochemical and genetic analysis of acyclovir-resistant mutants of herpes simplex virus type 1. Am J Med. 1982;73:351–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

