Synthetic Peptides as Protein Mimics
- PMID: 26835447
- PMCID: PMC4717299
- DOI: 10.3389/fbioe.2015.00211
Synthetic Peptides as Protein Mimics
Abstract
The design and generation of molecules capable of mimicking the binding and/or functional sites of proteins represents a promising strategy for the exploration and modulation of protein function through controlled interference with the underlying molecular interactions. Synthetic peptides have proven an excellent type of molecule for the mimicry of protein sites because such peptides can be generated as exact copies of protein fragments, as well as in diverse chemical modifications, which includes the incorporation of a large range of non-proteinogenic amino acids as well as the modification of the peptide backbone. Apart from extending the chemical and structural diversity presented by peptides, such modifications also increase the proteolytic stability of the molecules, enhancing their utility for biological applications. This article reviews recent advances by this and other laboratories in the use of synthetic protein mimics to modulate protein function, as well as to provide building blocks for synthetic biology.
Keywords: biomaterials; peptides; protein mimics; protein–protein interactions; structure-based design.
Figures
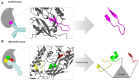
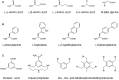
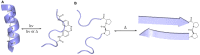
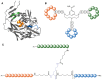

Similar articles
-
Peptides as protein binding site mimetics.Curr Opin Chem Biol. 2008 Dec;12(6):707-13. doi: 10.1016/j.cbpa.2008.09.023. Epub 2008 Oct 18. Curr Opin Chem Biol. 2008. PMID: 18935974 Review.
-
The RaPID Platform for the Discovery of Pseudo-Natural Macrocyclic Peptides.Acc Chem Res. 2021 Sep 21;54(18):3604-3617. doi: 10.1021/acs.accounts.1c00391. Epub 2021 Sep 10. Acc Chem Res. 2021. PMID: 34505781
-
The world of beta- and gamma-peptides comprised of homologated proteinogenic amino acids and other components.Chem Biodivers. 2004 Aug;1(8):1111-239. doi: 10.1002/cbdv.200490087. Chem Biodivers. 2004. PMID: 17191902 Review.
-
Macromolecular crowding: chemistry and physics meet biology (Ascona, Switzerland, 10-14 June 2012).Phys Biol. 2013 Aug;10(4):040301. doi: 10.1088/1478-3975/10/4/040301. Epub 2013 Aug 2. Phys Biol. 2013. PMID: 23912807
-
Sulfono-γ-AApeptides as Helical Mimetics: Crystal Structures and Applications.Acc Chem Res. 2020 Oct 20;53(10):2425-2442. doi: 10.1021/acs.accounts.0c00482. Epub 2020 Sep 17. Acc Chem Res. 2020. PMID: 32940995 Free PMC article.
Cited by
-
Ranacyclin-NF, a Novel Bowman-Birk Type Protease Inhibitor from the Skin Secretion of the East Asian Frog, Pelophylax nigromaculatus.Biology (Basel). 2020 Jul 2;9(7):149. doi: 10.3390/biology9070149. Biology (Basel). 2020. PMID: 32630758 Free PMC article.
-
Interferon-Stimulated Genes that Target Retrovirus Translation.Viruses. 2024 Jun 8;16(6):933. doi: 10.3390/v16060933. Viruses. 2024. PMID: 38932225 Free PMC article. Review.
-
Peptides to combat viral infectious diseases.Peptides. 2020 Dec;134:170402. doi: 10.1016/j.peptides.2020.170402. Epub 2020 Sep 1. Peptides. 2020. PMID: 32889022 Free PMC article. Review.
-
Angiogenin and Copper Crossing in Wound Healing.Int J Mol Sci. 2021 Oct 2;22(19):10704. doi: 10.3390/ijms221910704. Int J Mol Sci. 2021. PMID: 34639045 Free PMC article. Review.
-
Protein Mimetic and Anticancer Properties of Monocyte-Targeting Peptide Amphiphile Micelles.ACS Biomater Sci Eng. 2017 Dec 11;3(12):3273-3282. doi: 10.1021/acsbiomaterials.7b00600. Epub 2017 Sep 28. ACS Biomater Sci Eng. 2017. PMID: 29302619 Free PMC article.
References
-
- Aravinda S., Shamala N., Rajkishore R., Gopi H. N., Balaram P. (2002). A crystalline beta-hairpin peptide nucleated by a type I’ Aib-D-Ala beta-turn: evidence for cross-strand aromatic interactions. Angew. Chem. Int. Ed. Engl. 41, 3863–3865.10.1002/1521-3773(20021018)41:20<3863::AID-ANIE3863>3.0.CO;2-A - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

