Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria
- PMID: 26833236
- PMCID: PMC4852283
- DOI: 10.1093/femsre/fuw001
Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria
Abstract
Malaria accounts for an enormous burden of disease globally, with Plasmodium falciparum accounting for the majority of malaria, and P. vivax being a second important cause, especially in Asia, the Americas and the Pacific. During infection with Plasmodium spp., the merozoite form of the parasite invades red blood cells and replicates inside them. It is during the blood-stage of infection that malaria disease occurs and, therefore, understanding merozoite invasion, host immune responses to merozoite surface antigens, and targeting merozoite surface proteins and invasion ligands by novel vaccines and therapeutics have been important areas of research. Merozoite invasion involves multiple interactions and events, and substantial processing of merozoite surface proteins occurs before, during and after invasion. The merozoite surface is highly complex, presenting a multitude of antigens to the immune system. This complexity has proved challenging to our efforts to understand merozoite invasion and malaria immunity, and to developing merozoite antigens as malaria vaccines. In recent years, there has been major progress in this field, and several merozoite surface proteins show strong potential as malaria vaccines. Our current knowledge on this topic is reviewed, highlighting recent advances and research priorities.
Keywords: Plasmodium falciparum; Plasmodium vivax; antibodies; immunity; invasion; merozoites; vaccines.
© FEMS 2016.
Figures

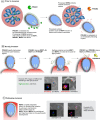
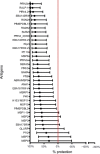

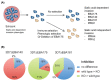
Similar articles
-
Identification of a potent combination of key Plasmodium falciparum merozoite antigens that elicit strain-transcending parasite-neutralizing antibodies.Infect Immun. 2013 Feb;81(2):441-51. doi: 10.1128/IAI.01107-12. Epub 2012 Nov 26. Infect Immun. 2013. PMID: 23184525 Free PMC article.
-
Parasite Recognition and Signaling Mechanisms in Innate Immune Responses to Malaria.Front Immunol. 2018 Dec 19;9:3006. doi: 10.3389/fimmu.2018.03006. eCollection 2018. Front Immunol. 2018. PMID: 30619355 Free PMC article. Review.
-
A library of functional recombinant cell-surface and secreted P. falciparum merozoite proteins.Mol Cell Proteomics. 2013 Dec;12(12):3976-86. doi: 10.1074/mcp.O113.028357. Epub 2013 Sep 16. Mol Cell Proteomics. 2013. PMID: 24043421 Free PMC article.
-
Parasite ligand-host receptor interactions during invasion of erythrocytes by Plasmodium merozoites.Int J Parasitol. 2004 Dec;34(13-14):1413-29. doi: 10.1016/j.ijpara.2004.10.010. Int J Parasitol. 2004. PMID: 15582519 Review.
-
Molecular interactions and signaling mechanisms during erythrocyte invasion by malaria parasites.Curr Opin Microbiol. 2011 Aug;14(4):422-8. doi: 10.1016/j.mib.2011.07.018. Epub 2011 Jul 29. Curr Opin Microbiol. 2011. PMID: 21803641 Review.
Cited by
-
Erythrocyte Adhesion of Merozoite Surface Antigen 2c1 Expressed During Extracellular Stages of Babesia orientalis.Front Immunol. 2021 May 17;12:623492. doi: 10.3389/fimmu.2021.623492. eCollection 2021. Front Immunol. 2021. PMID: 34079537 Free PMC article.
-
Dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria during pregnancy and risk of malaria in early childhood: A randomized controlled trial.PLoS Med. 2018 Jul 17;15(7):e1002606. doi: 10.1371/journal.pmed.1002606. eCollection 2018 Jul. PLoS Med. 2018. PMID: 30016328 Free PMC article. Clinical Trial.
-
Induction and Kinetics of Complement-Fixing Antibodies Against Plasmodium vivax Merozoite Surface Protein 3α and Relationship With Immunoglobulin G Subclasses and Immunoglobulin M.J Infect Dis. 2019 Nov 6;220(12):1950-1961. doi: 10.1093/infdis/jiz407. J Infect Dis. 2019. PMID: 31419296 Free PMC article.
-
Susceptibility to malaria in fulani, Bariba, Otamari and gando individuals living in sympatry in Benin: Role of opsonizing antibodies to Plasmodium falciparum merozoites.Heliyon. 2023 Jan 20;9(1):e13092. doi: 10.1016/j.heliyon.2023.e13092. eCollection 2023 Jan. Heliyon. 2023. PMID: 36711279 Free PMC article.
-
Clinical expression and antigenic profiles of a Plasmodium vivax vaccine candidate: merozoite surface protein 7 (PvMSP-7).Malar J. 2019 Jun 13;18(1):197. doi: 10.1186/s12936-019-2826-7. Malar J. 2019. PMID: 31196098 Free PMC article.
References
-
- Adams JH, Blair PL, Kaneko O, et al. An expanding ebl family of Plasmodium falciparum. Trends Parasitol. 2001;17:297–9. - PubMed
-
- Alonso PL, Smith T, Schellenberg JR, et al. Randomised trial of efficacy of SPf66 vaccine against Plasmodium falciparum malaria in children in southern Tanzania [see comments] Lancet. 1994;344:1175–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

