Dectin-1 Controls TLR9 Trafficking to Phagosomes Containing β-1,3 Glucan
- PMID: 26829985
- PMCID: PMC4761466
- DOI: 10.4049/jimmunol.1401545
Dectin-1 Controls TLR9 Trafficking to Phagosomes Containing β-1,3 Glucan
Abstract
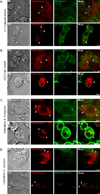
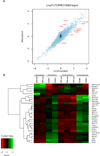
Dectin-1 and TLR9 play distinct roles in the recognition and induction of innate immune responses to Aspergillus fumigatus and Candida albicans. Dectin-1 is a receptor for the major fungal cell wall carbohydrate β-1,3 glucan that induces inflammatory cytokines and controls phagosomal maturation through spleen tyrosine kinase activation. TLR9 is an endosomal TLR that also modulates the inflammatory cytokine response to fungal pathogens. In this study, we demonstrate that β-1,3 glucan beads are sufficient to induce dynamic redistribution and accumulation of cleaved TLR9 to phagosomes. Trafficking of TLR9 to A. fumigatus and C. albicans phagosomes requires Dectin-1 recognition. Inhibition of phagosomal acidification blocks TLR9 accumulation on phagosomes containing β-1,3 glucan beads. Dectin-1-mediated spleen tyrosine kinase activation is required for TLR9 trafficking to β-1,3 glucan-, A. fumigatus-, and C. albicans-containing phagosomes. In addition, Dectin-1 regulates TLR9-dependent gene expression. Collectively, our study demonstrates that recognition of β-1,3 glucan by Dectin-1 triggers TLR9 trafficking to β-1,3 glucan-containing phagosomes, which may be critical in coordinating innate antifungal defense.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures
Similar articles
-
Dectin-1 activation controls maturation of β-1,3-glucan-containing phagosomes.J Biol Chem. 2013 May 31;288(22):16043-54. doi: 10.1074/jbc.M113.473223. Epub 2013 Apr 22. J Biol Chem. 2013. PMID: 23609446 Free PMC article.
-
TLR9 is actively recruited to Aspergillus fumigatus phagosomes and requires the N-terminal proteolytic cleavage domain for proper intracellular trafficking.J Immunol. 2010 Dec 15;185(12):7614-22. doi: 10.4049/jimmunol.1002760. Epub 2010 Nov 8. J Immunol. 2010. PMID: 21059889 Free PMC article.
-
Dectin-1-dependent LC3 recruitment to phagosomes enhances fungicidal activity in macrophages.J Infect Dis. 2014 Dec 1;210(11):1844-54. doi: 10.1093/infdis/jiu290. Epub 2014 May 19. J Infect Dis. 2014. PMID: 24842831 Free PMC article.
-
The role of Dectin-1 in the host defence against fungal infections.Curr Opin Microbiol. 2011 Aug;14(4):392-9. doi: 10.1016/j.mib.2011.07.001. Epub 2011 Jul 29. Curr Opin Microbiol. 2011. PMID: 21803640 Review.
-
β-Glucan signaling connects phagocytosis to autophagy.Glycobiology. 2013 Sep;23(9):1047-51. doi: 10.1093/glycob/cwt046. Epub 2013 Jun 6. Glycobiology. 2013. PMID: 23749474 Free PMC article. Review.
Cited by
-
It takes a village: Phagocytes play a central role in fungal immunity.Semin Cell Dev Biol. 2019 May;89:16-23. doi: 10.1016/j.semcdb.2018.04.008. Epub 2018 Jun 12. Semin Cell Dev Biol. 2019. PMID: 29727727 Free PMC article. Review.
-
Integrin-based diffusion barrier separates membrane domains enabling the formation of microbiostatic frustrated phagosomes.Elife. 2018 Mar 19;7:e34798. doi: 10.7554/eLife.34798. Elife. 2018. PMID: 29553370 Free PMC article.
-
Review: Impact of Helminth Infection on Antimycobacterial Immunity-A Focus on the Macrophage.Front Immunol. 2017 Dec 22;8:1864. doi: 10.3389/fimmu.2017.01864. eCollection 2017. Front Immunol. 2017. PMID: 29312343 Free PMC article. Review.
-
C-type lectin receptor-mediated immune recognition and response of the microbiota in the gut.Gastroenterol Rep (Oxf). 2019 Jul 10;7(5):312-321. doi: 10.1093/gastro/goz028. eCollection 2019 Oct. Gastroenterol Rep (Oxf). 2019. PMID: 31687150 Free PMC article. Review.
-
Postinfluenza Environment Reduces Aspergillus fumigatus Conidium Clearance and Facilitates Invasive Aspergillosis In Vivo.mBio. 2022 Dec 20;13(6):e0285422. doi: 10.1128/mbio.02854-22. Epub 2022 Nov 15. mBio. 2022. PMID: 36377895 Free PMC article.
References
-
- Miceli MH, Diaz JA, Lee SA. Emerging opportunistic yeast infections. Lancet Infect Dis. 2011;11:142–151. - PubMed
-
- Bozza S, Clavaud C, Giovannini G, Fontaine T, Beauvais A, Sarfati J, D’Angelo C, Perruccio K, Bonifazi P, Zagarella S, Moretti S, Bistoni F, Latge JP, Romani L. Immune sensing of Aspergillus fumigatus proteins, glycolipids, and polysaccharides and the impact on Th immunity and vaccination. J Immunol. 2009;183:2407–2414. - PubMed
-
- Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol. 2006;7:49–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

