Antibacterial Activity and Cytotoxicity of Silver(I) Complexes of Pyridine and (Benz)Imidazole Derivatives. X-ray Crystal Structure of [Ag(2,6-di(CH2OH)py)2]NO3
- PMID: 26828469
- PMCID: PMC6274122
- DOI: 10.3390/molecules21020087
Antibacterial Activity and Cytotoxicity of Silver(I) Complexes of Pyridine and (Benz)Imidazole Derivatives. X-ray Crystal Structure of [Ag(2,6-di(CH2OH)py)2]NO3
Abstract
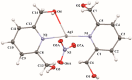
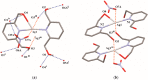
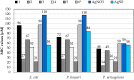
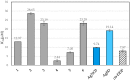
Selected aspects of the biological activity of a series of six nitrate silver(I) complexes with pyridine and (benz)imidazole derivatives were investigated. The present study evaluated the antibacterial activities of the complexes against three Gram-negative strains: Pseudomonas aeruginosa ATCC 15442, Escherichia coli ATCC 25922 and Proteus hauseri ATCC 13315. The results were compared with those of silver nitrate, a silver sulfadiazine drug and appropriate ligands. The most significant antibacterial properties were exerted by silver(I) complexes containing benzimidazole derivatives. The cytotoxic activity of the complexes was examined against B16 (murine melanoma) and 10T1/2 (murine fibroblasts) cells. All of the tested silver(I) compounds were not toxic to fibroblast cells in concentration inhibited cancer cell (B16) viability by 50%, which ranged between 2.44-28.65 µM. The molecular and crystal structure of silver(I) complex of 2,6-di(hydroxymethyl)pyridine was determined by single-crystal X-ray diffraction analysis. The most important features of the crystal packing and intermolecular non-covalent interactions in the Ag(I) complex were quantified via Hirshfeld surface analysis.
Keywords: 10T1/2 fibroblasts; B16 melanoma cells; Hirshfeld surface; antibacterial activity; cytotoxicity; silver(I) complexes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Synthesis, characterization and antimicrobial activity of water-soluble silver(i) complexes of metronidazole drug and selected counter-ions.Dalton Trans. 2015 May 7;44(17):8178-89. doi: 10.1039/c5dt00403a. Dalton Trans. 2015. PMID: 25846722
-
Sterically modulated silver(I) complexes of coumarin substituted benzimidazol-2-ylidenes: Synthesis, crystal structures and evaluation of their antimicrobial and antilung cancer potentials.J Inorg Biochem. 2018 Jun;183:43-57. doi: 10.1016/j.jinorgbio.2018.02.012. Epub 2018 Feb 22. J Inorg Biochem. 2018. PMID: 29529471
-
Synthesis, characterization, and antimicrobial activity of silver(I) and copper(II) complexes of phosphate derivatives of pyridine and benzimidazole.ChemMedChem. 2014 Jan;9(1):169-76. doi: 10.1002/cmdc.201300333. Epub 2013 Nov 11. ChemMedChem. 2014. PMID: 24218046
-
Silver Camphor Imine Complexes: Novel Antibacterial Compounds from Old Medicines.Antibiotics (Basel). 2018 Jul 26;7(3):65. doi: 10.3390/antibiotics7030065. Antibiotics (Basel). 2018. PMID: 30049958 Free PMC article. Review.
-
Essential Guide of Analysis Methods Applied to Silver Complexes with Antibacterial Quinolones.Adv Pharm Bull. 2018 Jun;8(2):181-189. doi: 10.15171/apb.2018.022. Epub 2018 Jun 19. Adv Pharm Bull. 2018. PMID: 30023319 Free PMC article. Review.
Cited by
-
Silver Sulfadiazine's Effect on Keratin-19 Expression as Stem Cell Marker in Burn Wound Healing.Biomedicine (Taipei). 2020 Jun 5;10(2):5-11. doi: 10.37796/2211-8039.1014. eCollection 2020. Biomedicine (Taipei). 2020. PMID: 33854915 Free PMC article.
-
Quinoline Functionalized Schiff Base Silver (I) Complexes: Interactions with Biomolecules and In Vitro Cytotoxicity, Antioxidant and Antimicrobial Activities.Molecules. 2021 Feb 24;26(5):1205. doi: 10.3390/molecules26051205. Molecules. 2021. PMID: 33668169 Free PMC article.
-
Multifunctional Silver(I) Complexes with Metronidazole Drug Reveal Antimicrobial Properties and Antitumor Activity against Human Hepatoma and Colorectal Adenocarcinoma Cells.Cancers (Basel). 2022 Feb 11;14(4):900. doi: 10.3390/cancers14040900. Cancers (Basel). 2022. PMID: 35205647 Free PMC article.
-
Silver, Its Salts and Application in Medicine and Pharmacy.Int J Mol Sci. 2023 Oct 29;24(21):15723. doi: 10.3390/ijms242115723. Int J Mol Sci. 2023. PMID: 37958707 Free PMC article. Review.
-
FSE-Ag complex NS: preparation and evaluation of antibacterial activity.IET Nanobiotechnol. 2018 Sep;12(6):836-840. doi: 10.1049/iet-nbt.2017.0284. IET Nanobiotechnol. 2018. PMID: 30104459 Free PMC article.
References
-
- Gholivand K., Molaei F., Oroujzadeh N., Mobasseri R., Naderi-Manesh H. Two novel Ag(I) complexes of N-nicotinyl phosphoric triamide derivatives: Synthesis, X-ray crystal structure and in vitro antibacterial and cytotoxicity studies. Inorg. Chim. Acta. 2014;423:107–116. doi: 10.1016/j.ica.2014.07.029. - DOI
-
- Kadayat T.M., Song C., Shin S., Magar T.B., Bist G., Shrestha A., Thapa P., Na Y., Kwon Y., Lee E.S. Synthesis, topoisomerase I and II inhibitory activity, cytotoxicity, and structure-activity relationship study of 2-phenyl- or hydroxylated 2-phenyl-4-aryl-5H-indeno[1,2-b]pyridines. Bioorg. Med. Chem. 2015;23:3499–3512. doi: 10.1016/j.bmc.2015.04.031. - DOI - PubMed
-
- Adhami F., Safavi M., Ehsani M., Ardestani S.K., Emmerling F., Simyari F. Synthesis, crystal structure, and cytotoxic activity of novel cyclic systems in [1,2,4]thiadiazolo[2,3-a]pyridine benzamide derivatives and their copper(II) complexes. Dalton Trans. 2014;43:7945–7957. doi: 10.1039/c3dt52905c. - DOI - PubMed
-
- Sun G.-X., Yang M.-Y., Shi Y.-X., Sun Z.-H., Liu X.-H., Wu H.-K., Li B.-J., Zhang Y.-G. Microwave assistant synthesis, antifungal activity and dft theoretical study of some novel 1,2,4-triazole derivatives containing pyridine moiety. Int. J. Mol. Sci. 2014;15:8075. doi: 10.3390/ijms15058075. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

