Cutting Edge: Critical Role of Glycolysis in Human Plasmacytoid Dendritic Cell Antiviral Responses
- PMID: 26826244
- PMCID: PMC4761472
- DOI: 10.4049/jimmunol.1501557
Cutting Edge: Critical Role of Glycolysis in Human Plasmacytoid Dendritic Cell Antiviral Responses
Abstract
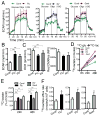
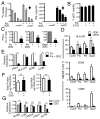
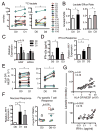
Plasmacytoid dendritic cells (pDCs) are vital to antiviral defense, directing immune responses via secretion of huge concentrations of IFN-α. These cells are critical in protecting the lung against clinically relevant respiratory viruses, particularly influenza (Flu), a virus responsible for substantial worldwide morbidity and mortality. How pDC responses to such viral pathogens are regulated, however, is poorly understood in humans. Using an unbiased approach of gene chip analysis, we discovered that Flu significantly affects metabolism in primary human pDCs. We demonstrate that Flu and RV, another common respiratory virus, induce glycolysis in pDCs and that this metabolic pathway regulates pDC antiviral functions, including IFN-α production and phenotypic maturation. Intranasal vaccination of human volunteers with live influenza virus also increases glycolysis in circulating pDCs, highlighting a previously unrecognized potential role for metabolism in regulating pDC immune responses to viral infections in humans.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures
Similar articles
-
Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells.J Immunol. 2010 Jun 1;184(11):5999-6006. doi: 10.4049/jimmunol.0901194. Epub 2010 Apr 21. J Immunol. 2010. PMID: 20410486 Free PMC article.
-
Human Blood and Tonsil Plasmacytoid Dendritic Cells Display Similar Gene Expression Profiles but Exhibit Differential Type I IFN Responses to Influenza A Virus Infection.J Immunol. 2019 Apr 1;202(7):2069-2081. doi: 10.4049/jimmunol.1801191. Epub 2019 Feb 13. J Immunol. 2019. PMID: 30760619
-
Differential responses of plasmacytoid dendritic cells to influenza virus and distinct viral pathogens.J Virol. 2014 Sep;88(18):10758-66. doi: 10.1128/JVI.01501-14. Epub 2014 Jul 9. J Virol. 2014. PMID: 25008918 Free PMC article.
-
CD28 Deficiency Enhances Type I IFN Production by Murine Plasmacytoid Dendritic Cells.J Immunol. 2016 Feb 15;196(4):1900-9. doi: 10.4049/jimmunol.1501658. Epub 2016 Jan 15. J Immunol. 2016. PMID: 26773151 Free PMC article.
-
Phthalates suppress type I interferon in human plasmacytoid dendritic cells via epigenetic regulation.Allergy. 2013 Jul;68(7):870-9. doi: 10.1111/all.12162. Epub 2013 Jun 5. Allergy. 2013. PMID: 23738920
Cited by
-
High ambient temperature dampens adaptive immune responses to influenza A virus infection.Proc Natl Acad Sci U S A. 2019 Feb 19;116(8):3118-3125. doi: 10.1073/pnas.1815029116. Epub 2019 Feb 4. Proc Natl Acad Sci U S A. 2019. PMID: 30718396 Free PMC article.
-
Metabolic Adaptations to Infections at the Organismal Level.Trends Immunol. 2020 Feb;41(2):113-125. doi: 10.1016/j.it.2019.12.001. Epub 2020 Jan 17. Trends Immunol. 2020. PMID: 31959515 Free PMC article. Review.
-
Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab.J Allergy Clin Immunol. 2018 May;141(5):1735-1743.e9. doi: 10.1016/j.jaci.2017.07.035. Epub 2017 Sep 1. J Allergy Clin Immunol. 2018. PMID: 28870461 Free PMC article. Clinical Trial.
-
Glucose Metabolism in T Cells and Monocytes: New Perspectives in HIV Pathogenesis.EBioMedicine. 2016 Apr;6:31-41. doi: 10.1016/j.ebiom.2016.02.012. Epub 2016 Feb 6. EBioMedicine. 2016. PMID: 27211546 Free PMC article. Review.
-
Type I Interferon Induction and Exhaustion during Viral Infection: Plasmacytoid Dendritic Cells and Emerging COVID-19 Findings.Viruses. 2021 Sep 15;13(9):1839. doi: 10.3390/v13091839. Viruses. 2021. PMID: 34578420 Free PMC article. Review.
References
-
- Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. Jama. 2004;292:1333–1340. - PubMed
-
- Gill MA, Long K, Kwon T, Muniz L, Mejias A, Connolly J, Roy L, Banchereau J, Ramilo O. Differential recruitment of dendritic cells and monocytes to respiratory mucosal sites in children with influenza virus or respiratory syncytial virus infection. The Journal of infectious diseases. 2008;198:1667–1676. - PMC - PubMed
-
- Kaminski MM, Ohnemus A, Cornitescu M, Staeheli P. Plasmacytoid dendritic cells and Toll-like receptor 7-dependent signalling promote efficient protection of mice against highly virulent influenza A virus. The Journal of general virology. 2012;93:555–559. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

