Discovery of a Potent and Orally Efficacious TGR5 Receptor Agonist
- PMID: 26819665
- PMCID: PMC4716599
- DOI: 10.1021/acsmedchemlett.5b00323
Discovery of a Potent and Orally Efficacious TGR5 Receptor Agonist
Abstract
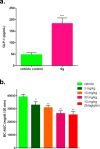
TGR5 is a G protein-coupled receptor (GPCR), activation of which promotes secretion of glucagon-like peptide-1 (GLP-1) and modulates insulin secretion. The 2-thio-imidazole derivative 6g was identified as a novel, potent, and selective TGR5 agonist (hTGR5 EC50 = 57 pM, mTGR5 = 62 pM) with a favorable pharmacokinetic profile. The compound 6g was found to have potent glucose lowering effects in vivo during an oral glucose tolerance test in DIO C57 mice with ED50 of 7.9 mg/kg and ED90 of 29.2 mg/kg.
Keywords: GLP1 secretion; GPBAR1; GPR 131; TGR5; TGR5 agonist; diabetes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Evaluation of novel TGR5 agonist in combination with Sitagliptin for possible treatment of type 2 diabetes.Bioorg Med Chem Lett. 2018 Jun 1;28(10):1849-1852. doi: 10.1016/j.bmcl.2018.04.011. Epub 2018 Apr 5. Bioorg Med Chem Lett. 2018. PMID: 29655980
-
TGR5 agonist ameliorates insulin resistance in the skeletal muscles and improves glucose homeostasis in diabetic mice.Metabolism. 2019 Oct;99:45-56. doi: 10.1016/j.metabol.2019.07.003. Epub 2019 Jul 8. Metabolism. 2019. PMID: 31295453
-
Design, synthesis, and antidiabetic activity of 4-phenoxynicotinamide and 4-phenoxypyrimidine-5-carboxamide derivatives as potent and orally efficacious TGR5 agonists.J Med Chem. 2012 Dec 13;55(23):10475-89. doi: 10.1021/jm301071h. Epub 2012 Nov 29. J Med Chem. 2012. PMID: 23148522
-
Crosstalk between FXR and TGR5 controls glucagon-like peptide 1 secretion to maintain glycemic homeostasis.Lab Anim Res. 2018 Dec;34(4):140-146. doi: 10.5625/lar.2018.34.4.140. Epub 2018 Dec 31. Lab Anim Res. 2018. PMID: 30671099 Free PMC article. Review.
-
Clinical relevance of the bile acid receptor TGR5 in metabolism.Lancet Diabetes Endocrinol. 2017 Mar;5(3):224-233. doi: 10.1016/S2213-8587(16)30155-3. Epub 2016 Sep 14. Lancet Diabetes Endocrinol. 2017. PMID: 27639537 Review.
Cited by
-
Investigation of triamterene as an inhibitor of the TGR5 receptor: identification in cells and animals.Drug Des Devel Ther. 2017 Apr 5;11:1127-1134. doi: 10.2147/DDDT.S131892. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28435224 Free PMC article.
-
TGR5, Not Only a Metabolic Regulator.Front Physiol. 2016 Dec 26;7:646. doi: 10.3389/fphys.2016.00646. eCollection 2016. Front Physiol. 2016. PMID: 28082913 Free PMC article. Review.
-
Improving glucose and lipids metabolism: drug development based on bile acid related targets.Cell Stress. 2021 Jan 5;5(1):1-18. doi: 10.15698/cst2021.01.239. Cell Stress. 2021. PMID: 33447732 Free PMC article. Review.
-
Ursolic acid activates the TGR5 receptor to enhance GLP-1 secretion in type 1-like diabetic rats.Naunyn Schmiedebergs Arch Pharmacol. 2017 Nov;390(11):1097-1104. doi: 10.1007/s00210-017-1409-9. Epub 2017 Jul 30. Naunyn Schmiedebergs Arch Pharmacol. 2017. PMID: 28756460
-
Differential action of TGR5 agonists on GLP-2 secretion and promotion of intestinal adaptation in a piglet short bowel model.Am J Physiol Gastrointest Liver Physiol. 2019 May 1;316(5):G641-G652. doi: 10.1152/ajpgi.00360.2018. Epub 2019 Mar 28. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 30920308 Free PMC article.
References
-
- IBD Diabetes Atlas, 6th ed.; International Diabetes Federation: Brussells, Belgium, 2013; http://www.diabetesatlas.org.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

