Development and First Validation of a Disease Activity Score for Gout
- PMID: 26815286
- PMCID: PMC5129490
- DOI: 10.1002/acr.22844
Development and First Validation of a Disease Activity Score for Gout
Abstract
Objective: To develop a new composite disease activity score for gout and provide its first validation.
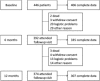
Methods: Disease activity has been defined as the ongoing presence of urate deposits that lead to acute arthritis and joint damage. Every measure for each Outcome Measures in Rheumatology core domain was considered. A 3-step approach (factor analysis, linear discriminant analysis, and linear regression) was applied to derive the Gout Activity Score (GAS). Decision to change treatment or 6-month flare count were used as the surrogate criteria of high disease activity. Baseline and 12-month followup data of 446 patients included in the Kick-Off of the Italian Network for Gout cohort were used. Construct- and criterion-related validity were tested. External validation on an independent sample is reported.
Results: Factor analysis identified 5 factors: patient-reported outcomes, joint examination, flares, tophi, and serum uric acid (sUA). Discriminant function analysis resulted in a correct classification of 79%. Linear regression analysis identified a first candidate GAS including 12-month flare count, sUA, visual analog scale (VAS) of pain, VAS global activity assessment, swollen and tender joint counts, and a cumulative measure of tophi. Alternative scores were also developed. The developed GAS demonstrated a good correlation with functional disability (criterion validity) and discrimination between patient- and physician-reported measures of active disease (construct validity). The results were reproduced in the external sample.
Conclusion: This study developed and validated a composite measure of disease activity in gout. Further testing is required to confirm its generalizability, responsiveness, and usefulness in assisting with clinical decisions.
© 2016, The Authors. Arthritis Care & Research published by Wiley Periodicals, Inc. on behalf of the American College of Rheumatology.
Figures
Similar articles
-
Predictors of disease activity in gout: a 12-month analysis of the ATTACk (Achieving improvement in the management of crystal-induced arthritis) multicentre cohort study.Clin Exp Rheumatol. 2023 Mar;41(3):628-633. doi: 10.55563/clinexprheumatol/eh0jcp. Epub 2022 Aug 5. Clin Exp Rheumatol. 2023. PMID: 35930471
-
A world of hurt: failure to achieve treatment goals in patients with gout requires a paradigm shift.Postgrad Med. 2016 Jan;128(1):34-40. doi: 10.1080/00325481.2016.1113840. Epub 2015 Nov 17. Postgrad Med. 2016. PMID: 26578028
-
Foot pain, impairment, and disability in patients with acute gout flares: A prospective observational study.Arthritis Care Res (Hoboken). 2012 Mar;64(3):384-8. doi: 10.1002/acr.20670. Arthritis Care Res (Hoboken). 2012. PMID: 22006512
-
Overview of Serum Uric Acid Treatment Targets in Gout: Why Less Than 6 mg/dL?Postgrad Med. 2016 Sep;128(7):706-15. doi: 10.1080/00325481.2016.1221732. Epub 2016 Aug 25. Postgrad Med. 2016. PMID: 27558643 Review.
-
Serum urate as a soluble biomarker in chronic gout-evidence that serum urate fulfills the OMERACT validation criteria for soluble biomarkers.Semin Arthritis Rheum. 2011 Jun;40(6):483-500. doi: 10.1016/j.semarthrit.2010.09.003. Epub 2010 Nov 5. Semin Arthritis Rheum. 2011. PMID: 21056459 Review.
Cited by
-
Concurrent validity of provisional remission criteria for gout: a dual-energy CT study.Arthritis Res Ther. 2019 Jun 21;21(1):150. doi: 10.1186/s13075-019-1941-8. Arthritis Res Ther. 2019. PMID: 31227018 Free PMC article.
-
Gout Activity Score has predictive validity and is sensitive to change: results from the Nottingham Gout Treatment Trial (Phase II).Rheumatology (Oxford). 2019 Feb 4;58(8):1378-82. doi: 10.1093/rheumatology/key446. Online ahead of print. Rheumatology (Oxford). 2019. PMID: 30726962 Free PMC article.
-
Sonographic estimation of monosodium urate burden predicts the fulfillment of the 2016 remission criteria for gout: a 12-month study.Arthritis Res Ther. 2021 Jul 9;23(1):185. doi: 10.1186/s13075-021-02568-x. Arthritis Res Ther. 2021. PMID: 34243813 Free PMC article.
-
Development of a multivariable improvement measure for gout.Arthritis Res Ther. 2020 Jun 29;22(1):164. doi: 10.1186/s13075-020-02254-4. Arthritis Res Ther. 2020. PMID: 32600452 Free PMC article.
-
What Is the Evidence for Treat-to-Target Serum Urate in Gout?Curr Rheumatol Rep. 2018 Mar 8;20(3):11. doi: 10.1007/s11926-018-0719-3. Curr Rheumatol Rep. 2018. PMID: 29516287 Review.
References
-
- Stamp LK, Zhu X, Dalbeth N, Jordan S, Edwards NL, Taylor W. Serum urate as a soluble biomarker in chronic gout: evidence that serum urate fulfills the OMERACT validation criteria for soluble biomarkers. Semin Arthritis Rheum 2011;40:483–500. - PubMed
-
- Taylor WJ, Brown M, Aati O, Weatherall M, Dalbeth N. Do patient preferences for core outcome domains for chronic gout studies support the validity of composite response criteria? Arthritis Care Res (Hoboken) 2013;65:1259–64. - PubMed
-
- Perez‐Ruiz F. Treating to target: a strategy to cure gout. Rheumatology (Oxford) 2009;48:ii9–14. - PubMed
-
- Taylor WJ, Singh JA, Saag KG, Dalbeth N, MacDonald PA, Edwards NL, et al. Bringing it all together: a novel approach to the development of response criteria for chronic gout clinical trials. J Rheumatol 2011;38:1467–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical