Radiolabeling and evaluation of (64)Cu-DOTA-F56 peptide targeting vascular endothelial growth factor receptor 1 in the molecular imaging of gastric cancer
- PMID: 26807312
- PMCID: PMC4697678
Radiolabeling and evaluation of (64)Cu-DOTA-F56 peptide targeting vascular endothelial growth factor receptor 1 in the molecular imaging of gastric cancer
Abstract
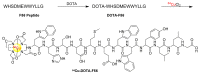
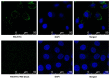
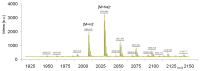
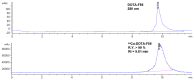
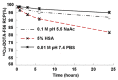
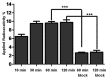
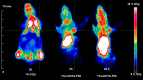
Noninvasive imaging of vascular endothelial growth factor receptor 1 (VEGFR1) remains a great challenge in early diagnosis of gastric cancer. Here we reported the synthesis, radiolabeling, and evaluation of a novel (64)Cu-radiolabeled peptide for noninvasive positron emission tomography (PET) imaging of VEGFR1 positive gastric cancer. The binding of modified peptide WHSDMEWWYLLG (termed as F56) to VEGER-1 expressed in gastric cancer cell BCG823 has been confirmed by immune-fluorescence overlap. DOTA-F56 was designed and prepared by solid-phase synthesis and folded in vitro. (64)Cu-DOTA-F56 was synthesized in high radiochemical yield and high specific activity (S.A. up to 255.6 GBq/mmol). It has excellent in vitro stability. Micro-PET imaging of (64)Cu-DOTA-F56 identifies tumor in BCG823 tumor-bearing mice, while that of (18)F-FDG does not. Immunohistochemical analysis of excised BCG823 xenograft showed colocalization between the PET images and the staining of VEGFR1. These results demonstrated that (64)Cu-DOTA-F56 peptide has potential as a noninvasive imaging agent in VEGFR1 positive tumors.
Keywords: F56 peptide; Vascular endothelial growth factor receptor 1; copper-64; gastric cancer; molecular imaging.
Figures

Similar articles
-
125I-F56 Peptide as Radioanalysis Agent Targeting VEGFR1 in Mice Xenografted with Human Gastric Tumor.ACS Med Chem Lett. 2017 Jan 20;8(2):266-269. doi: 10.1021/acsmedchemlett.6b00498. eCollection 2017 Feb 9. ACS Med Chem Lett. 2017. PMID: 28197324 Free PMC article.
-
Evaluation of 111In-DOTA-F56 peptide targeting VEGFR1 for potential non-invasive gastric cancer xenografted tumor mice Micro-SPECT imaging.Bioorg Med Chem Lett. 2020 Jul 15;30(14):127248. doi: 10.1016/j.bmcl.2020.127248. Epub 2020 May 8. Bioorg Med Chem Lett. 2020. PMID: 32527549
-
PET Imaging of HER2-Positive Tumors with Cu-64-Labeled Affibody Molecules.Mol Imaging Biol. 2019 Oct;21(5):907-916. doi: 10.1007/s11307-018-01310-5. Mol Imaging Biol. 2019. PMID: 30617730
-
PET of vascular endothelial growth factor receptor expression.J Nucl Med. 2006 Dec;47(12):2048-56. J Nucl Med. 2006. PMID: 17138749
-
A VEGFR1 antagonistic peptide inhibits tumor growth and metastasis through VEGFR1-PI3K-AKT signaling pathway inhibition.Am J Cancer Res. 2015 Sep 15;5(10):3149-61. eCollection 2015. Am J Cancer Res. 2015. PMID: 26693066 Free PMC article.
Cited by
-
ImmunoPET imaging of CD38 expression in hepatocellular carcinoma using 64Cu-labeled daratumumab.Am J Transl Res. 2019 Sep 15;11(9):6007-6015. eCollection 2019. Am J Transl Res. 2019. PMID: 31632568 Free PMC article.
-
Development of a novel albumin-based and maleimidopropionic acid-conjugated peptide with prolonged half-life and increased in vivo anti-tumor efficacy.Theranostics. 2018 Mar 7;8(8):2094-2106. doi: 10.7150/thno.22069. eCollection 2018. Theranostics. 2018. PMID: 29721065 Free PMC article.
-
125I-F56 Peptide as Radioanalysis Agent Targeting VEGFR1 in Mice Xenografted with Human Gastric Tumor.ACS Med Chem Lett. 2017 Jan 20;8(2):266-269. doi: 10.1021/acsmedchemlett.6b00498. eCollection 2017 Feb 9. ACS Med Chem Lett. 2017. PMID: 28197324 Free PMC article.
-
Molecular imaging of Toll-like receptor 4 detects ischemia-reperfusion injury during intussusception.Oncotarget. 2017 Dec 22;9(8):7882-7890. doi: 10.18632/oncotarget.23609. eCollection 2018 Jan 30. Oncotarget. 2017. PMID: 29487699 Free PMC article.
-
Vascular endothelial growth receptor 1 acts as a stress-associated protein in the therapeutic response to thalidomide.Exp Ther Med. 2017 Nov;14(5):4263-4271. doi: 10.3892/etm.2017.5028. Epub 2017 Aug 24. Exp Ther Med. 2017. PMID: 29075340 Free PMC article.
References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U, Gotoda T, Lin JT, You WC, Ng EK, Sung JJ. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279–287. - PubMed
-
- Yu M, Zheng HC, Xia P, Takahashi H, Masuda S, Takano Y, Xu HM. Comparison in pathological behaviours & prognosis of gastric cancers from general hospitals between China & Japan. Indian J Med Res. 2010;132:295–302. - PubMed
-
- Shou CC, Wu AW. [Application of biotherapy in recurrent or metastatic gastric cancer] . Zhonghua Wei Chang Wai Ke Za Zhi. 2011;14:569–572. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
