The role of the Annexin-A1/FPR2 system in the regulation of mast cell degranulation provoked by compound 48/80 and in the inhibitory action of nedocromil
- PMID: 26803520
- PMCID: PMC4760273
- DOI: 10.1016/j.intimp.2016.01.003
The role of the Annexin-A1/FPR2 system in the regulation of mast cell degranulation provoked by compound 48/80 and in the inhibitory action of nedocromil
Abstract
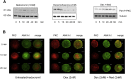
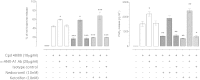
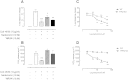
1.We investigated the role of Annexin (ANX)-A1 and its receptor, ALX/FPR2, in the regulation of mast cell degranulation produced by compound 48/80. 2.Both human cord-blood derived mast cells (CBDMCs) and murine bone marrow derived mast cells (BMDMCs) release phosphorylated ANX-A1 during treatment with glucocorticoids or the mast cell 'stabilising' drugs ketotifen and nedocromil. 3.Compound 48/80 also stimulated ANX-A1 phosphorylation and release and this was also potentiated by nedocromil. Anti-ANX-A1 neutralising monoclonal antibodies (Mabs) enhanced the release of pro-inflammatory mediators in response to compound 48/80. 4.Nedocromil and ketotifen potently inhibited the release of histamine, PGD2, tryptase and β-hexosaminidase from mast cells challenged with compound 48/80. Anti-ANX-A1 neutralising Mabs prevented the inhibitory effect of these drugs. 5.BMDMCs derived from Anx-A1−/− mice were insensitive to the inhibitory effects of nedocromil or ketotifen but cells retained their sensitivity to the inhibitory action of hu-r-ANX-A1. 6.The fpr2/3 antagonist WRW4 blocked the action of nedocromil on PGD2, but not histamine, release. BMDMCs derived from fpr2/3−/− mice were insensitive to the inhibitory effects of nedocromil on PGD2, but not histamine release. 7.Compound 48/80 stimulated both p38 and JNK phosphorylation in CBDMCs and this was inhibited by nedocromil. Inhibition of p38 phosphorylation was ANX-A1 dependent. 8.We conclude that ANX-A1 is an important regulator of mast cell reactivity to compound 48/80 exerting a negative feedback effect through a mechanism that depends at least partly on the FPR receptor.
Keywords: ANX-A1; Compound 48/80; Cromones; Mast cell; PKC; PP2A.
Figures



Similar articles
-
Anti-allergic cromones inhibit histamine and eicosanoid release from activated human and murine mast cells by releasing Annexin A1.PLoS One. 2013;8(3):e58963. doi: 10.1371/journal.pone.0058963. Epub 2013 Mar 18. PLoS One. 2013. PMID: 23527056 Free PMC article.
-
Antiallergic cromones inhibit neutrophil recruitment onto vascular endothelium via annexin-A1 mobilization.Arterioscler Thromb Vasc Biol. 2010 Sep;30(9):1718-24. doi: 10.1161/ATVBAHA.110.209536. Epub 2010 Jun 17. Arterioscler Thromb Vasc Biol. 2010. PMID: 20558817 Free PMC article.
-
Inflammation and cancer: role of annexin A1 and FPR2/ALX in proliferation and metastasis in human laryngeal squamous cell carcinoma.PLoS One. 2014 Dec 9;9(12):e111317. doi: 10.1371/journal.pone.0111317. eCollection 2014. PLoS One. 2014. PMID: 25490767 Free PMC article.
-
Anti-inflammatory drugs, eicosanoids and the annexin A1/FPR2 anti-inflammatory system.Prostaglandins Other Lipid Mediat. 2012 Aug;98(3-4):94-100. doi: 10.1016/j.prostaglandins.2011.11.005. Epub 2011 Nov 23. Prostaglandins Other Lipid Mediat. 2012. PMID: 22123264 Review.
-
[Membrane stabilizers (chromones and ketotifen)].Allerg Immunol (Paris). 1999 Apr;31(4):103-5. Allerg Immunol (Paris). 1999. PMID: 10370721 Review. French.
Cited by
-
Annexin Animal Models-From Fundamental Principles to Translational Research.Int J Mol Sci. 2021 Mar 26;22(7):3439. doi: 10.3390/ijms22073439. Int J Mol Sci. 2021. PMID: 33810523 Free PMC article. Review.
-
Endogenous Annexin-A1 Negatively Regulates Mast Cell-Mediated Allergic Reactions.Front Pharmacol. 2019 Nov 13;10:1313. doi: 10.3389/fphar.2019.01313. eCollection 2019. Front Pharmacol. 2019. PMID: 31798445 Free PMC article.
-
Triple-Negative Breast Cancer with High Levels of Annexin A1 Expression Is Associated with Mast Cell Infiltration, Inflammation, and Angiogenesis.Int J Mol Sci. 2019 Aug 27;20(17):4197. doi: 10.3390/ijms20174197. Int J Mol Sci. 2019. PMID: 31461932 Free PMC article.
-
The potential of lipid mediator networks as ocular surface therapeutics and biomarkers.Ocul Surf. 2021 Jan;19:104-114. doi: 10.1016/j.jtos.2020.04.008. Epub 2020 Apr 29. Ocul Surf. 2021. PMID: 32360792 Free PMC article. Review.
-
Annexin A1: shifting the balance towards resolution and repair.Biol Chem. 2016 Oct 1;397(10):971-9. doi: 10.1515/hsz-2016-0180. Biol Chem. 2016. PMID: 27232634 Free PMC article. Review.
References
-
- Perretti M., D'Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 2009;9:62–70. - PubMed
-
- Browning J.L., Ward M.P., Wallner B.P., Pepinsky R.B. Studies on the structural properties of lipocortin-1 and the regulation of its synthesis by steroids. Prog. Clin. Biol. Res. 1990;349:27–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

