Pathophysiology of the cochlear intrastrial fluid-blood barrier (review)
- PMID: 26802581
- PMCID: PMC5322264
- DOI: 10.1016/j.heares.2016.01.010
Pathophysiology of the cochlear intrastrial fluid-blood barrier (review)
Abstract
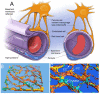
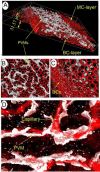
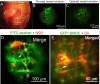
The blood-labyrinth barrier (BLB) in the stria vascularis is a highly specialized capillary network that controls exchanges between blood and the intrastitial space in the cochlea. The barrier shields the inner ear from blood-born toxic substances and selectively passes ions, fluids, and nutrients to the cochlea, playing an essential role in the maintenance of cochlear homeostasis. Anatomically, the BLB is comprised of endothelial cells (ECs) in the strial microvasculature, elaborated tight and adherens junctions, pericytes (PCs), basement membrane (BM), and perivascular resident macrophage-like melanocytes (PVM/Ms), which together form a complex "cochlear-vascular unit" in the stria vascularis. Physical interactions between the ECs, PCs, and PVM/Ms, as well as signaling between the cells, is critical for controlling vascular permeability and providing a proper environment for hearing function. Breakdown of normal interactions between components of the BLB is seen in a wide range of pathological conditions, including genetic defects and conditions engendered by inflammation, loud sound trauma, and ageing. In this review, we will discuss prevailing views of the structure and function of the strial cochlear-vascular unit (also referred to as the "intrastrial fluid-blood barrier"). We will also discuss the disrupted homeostasis seen in a variety of hearing disorders. Therapeutic targeting of the strial barrier may offer opportunities for improvement of hearing health and amelioration of auditory disorders. This article is part of a Special Issue entitled <Annual Reviews 2016>.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures




Similar articles
-
Endothelial cell, pericyte, and perivascular resident macrophage-type melanocyte interactions regulate cochlear intrastrial fluid-blood barrier permeability.J Assoc Res Otolaryngol. 2013 Apr;14(2):175-85. doi: 10.1007/s10162-012-0365-9. Epub 2012 Dec 18. J Assoc Res Otolaryngol. 2013. PMID: 23247886 Free PMC article.
-
Perivascular macrophage-like melanocyte responsiveness to acoustic trauma--a salient feature of strial barrier associated hearing loss.FASEB J. 2013 Sep;27(9):3730-40. doi: 10.1096/fj.13-232892. Epub 2013 May 31. FASEB J. 2013. PMID: 23729595 Free PMC article.
-
Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid-blood barrier.Proc Natl Acad Sci U S A. 2012 Jun 26;109(26):10388-93. doi: 10.1073/pnas.1205210109. Epub 2012 Jun 11. Proc Natl Acad Sci U S A. 2012. PMID: 22689949 Free PMC article.
-
Cochlear Capillary Pericytes.Adv Exp Med Biol. 2019;1122:115-123. doi: 10.1007/978-3-030-11093-2_7. Adv Exp Med Biol. 2019. PMID: 30937866 Review.
-
A critical evaluation of "leakage" at the cochlear blood-stria-barrier and its functional significance.Front Mol Neurosci. 2024 Feb 29;17:1368058. doi: 10.3389/fnmol.2024.1368058. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38486963 Free PMC article. Review.
Cited by
-
Intratympanic Lipopolysaccharide Elevates Systemic Fluorescent Gentamicin Uptake in the Cochlea.Laryngoscope. 2021 Sep;131(9):E2573-E2582. doi: 10.1002/lary.29610. Epub 2021 May 6. Laryngoscope. 2021. PMID: 33956344 Free PMC article.
-
Effects of Diet and Lifestyle on Audio-Vestibular Dysfunction in the Elderly: A Literature Review.Nutrients. 2022 Nov 8;14(22):4720. doi: 10.3390/nu14224720. Nutrients. 2022. PMID: 36432406 Free PMC article. Review.
-
[The distribution of perivascular-resident cells in blood-labyrinth barrier observed with two-photon fluorescence microscope and Imaris deconvolution].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020 Jun;34(6):486-491. doi: 10.13201/j.issn.2096-7993.2020.06.002. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020. PMID: 32842175 Free PMC article. Chinese.
-
Tumor necrosis factor-α mediated inflammation versus apoptosis in age-related hearing loss.Front Aging Neurosci. 2022 Sep 7;14:956503. doi: 10.3389/fnagi.2022.956503. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36158549 Free PMC article.
-
Update on Findings about Sudden Sensorineural Hearing Loss and Insight into Its Pathogenesis.J Clin Med. 2022 Oct 28;11(21):6387. doi: 10.3390/jcm11216387. J Clin Med. 2022. PMID: 36362614 Free PMC article. Review.
References
-
- Adamson R. Role of macrophages in normal wound healing: an overview. J Wound Care. 2009;18:349–351. - PubMed
-
- Ågrup C, Luxon LM. Immune-mediated inner-ear disorders in neuro-otology. Curr Opin Neurol. 2006;19:26–32. - PubMed
-
- Allt G, Lawrenson J. Pericytes: cell biology and pathology. Cells Tissues Organs. 2001;169:1–11. - PubMed
-
- Balabanov R, Dore-Duffy P. Role of the CNS microvascular pericyte in the blood-brain barrier. J Neurosci Res. 1998;53:637–644. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

