TSH Receptor Cleavage Into Subunits and Shedding of the A-Subunit; A Molecular and Clinical Perspective
- PMID: 26799472
- PMCID: PMC4823380
- DOI: 10.1210/er.2015-1098
TSH Receptor Cleavage Into Subunits and Shedding of the A-Subunit; A Molecular and Clinical Perspective
Retraction in
-
Erratum Notice for Duplicate Publications.Endocr Rev. 2020 Dec 1;41(6):886. doi: 10.1210/endrev/bnaa019. Endocr Rev. 2020. PMID: 32805736 Free PMC article. No abstract available.
Abstract
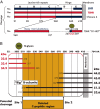
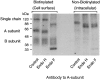
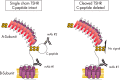
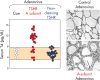
The TSH receptor (TSHR) on the surface of thyrocytes is unique among the glycoprotein hormone receptors in comprising two subunits: an extracellular A-subunit, and a largely transmembrane and cytosolic B-subunit. Unlike its ligand TSH, whose subunits are encoded by two genes, the TSHR is expressed as a single polypeptide that subsequently undergoes intramolecular cleavage into disulfide-linked subunits. Cleavage is associated with removal of a C-peptide region, a mechanism similar in some respects to insulin cleavage into disulfide linked A- and B-subunits with loss of a C-peptide region. The potential pathophysiological importance of TSHR cleavage into A- and B-subunits is that some A-subunits are shed from the cell surface. Considerable experimental evidence supports the concept that A-subunit shedding in genetically susceptible individuals is a factor contributing to the induction and/or affinity maturation of pathogenic thyroid-stimulating autoantibodies, the direct cause of Graves' disease. The noncleaving gonadotropin receptors are not associated with autoantibodies that induce a "Graves' disease of the gonads." We also review herein current information on the location of the cleavage sites, the enzyme(s) responsible for cleavage, the mechanism by which A-subunits are shed, and the effects of cleavage on receptor signaling.
Figures

Similar articles
-
Withdrawn: TSH Receptor Cleavage Into Subunits and Shedding of the A-Subunit; A Molecular and Clinical Perspective.Endocr Rev. 2016 Feb;2016(1):23-42. doi: 10.1210/er.2015-1098.2016.1.test. Endocr Rev. 2016. Retraction in: Endocr Rev. 2020 Dec 1;41(6):bnaa019. doi: 10.1210/endrev/bnaa019. PMID: 27454362 Free PMC article. Retracted. Review.
-
Evidence that TSH Receptor A-Subunit Multimers, Not Monomers, Drive Antibody Affinity Maturation in Graves' Disease.J Clin Endocrinol Metab. 2015 Jun;100(6):E871-5. doi: 10.1210/jc.2015-1528. Epub 2015 Apr 9. J Clin Endocrinol Metab. 2015. PMID: 25856215 Free PMC article.
-
Subunit interactions influence TSHR multimerization.Mol Endocrinol. 2010 Oct;24(10):2009-18. doi: 10.1210/me.2010-0001. Epub 2010 Aug 18. Mol Endocrinol. 2010. PMID: 20719860 Free PMC article.
-
A Mouse Thyrotropin Receptor A-Subunit Transgene Expressed in Thyroiditis-Prone Mice May Provide Insight into Why Graves' Disease Only Occurs in Humans.Thyroid. 2019 Aug;29(8):1138-1146. doi: 10.1089/thy.2019.0260. Epub 2019 Jul 3. Thyroid. 2019. PMID: 31184281 Free PMC article.
-
Graves' Disease Mechanisms: The Role of Stimulating, Blocking, and Cleavage Region TSH Receptor Antibodies.Horm Metab Res. 2015 Sep;47(10):727-34. doi: 10.1055/s-0035-1559633. Epub 2015 Sep 11. Horm Metab Res. 2015. PMID: 26361259 Free PMC article. Review.
Cited by
-
The Molecular Function and Clinical Role of Thyroid Stimulating Hormone Receptor in Cancer Cells.Cells. 2020 Jul 20;9(7):1730. doi: 10.3390/cells9071730. Cells. 2020. PMID: 32698392 Free PMC article. Review.
-
Thyroid Autoantibodies Display both "Original Antigenic Sin" and Epitope Spreading.Front Immunol. 2017 Dec 20;8:1845. doi: 10.3389/fimmu.2017.01845. eCollection 2017. Front Immunol. 2017. PMID: 29326719 Free PMC article. Review.
-
High-level intrathymic thyrotrophin receptor expression in thyroiditis-prone mice protects against the spontaneous generation of pathogenic thyrotrophin receptor autoantibodies.Clin Exp Immunol. 2017 May;188(2):243-253. doi: 10.1111/cei.12928. Epub 2017 Feb 20. Clin Exp Immunol. 2017. PMID: 28099999 Free PMC article.
-
Thyrotropin Receptor Epitope and Human Leukocyte Antigen in Graves' Disease.Front Endocrinol (Lausanne). 2016 Aug 23;7:120. doi: 10.3389/fendo.2016.00120. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 27602020 Free PMC article. Review.
-
A review of TSHR- and IGF-1R-related pathogenesis and treatment of Graves' orbitopathy.Front Immunol. 2023 Jan 19;14:1062045. doi: 10.3389/fimmu.2023.1062045. eCollection 2023. Front Immunol. 2023. PMID: 36742308 Free PMC article. Review.
References
-
- Amir SM, Carraway TF, Jr, Kohn LD, Winand RJ. The binding of thyrotropin to isolated bovine thyroid plasma membranes. J Biol Chem. 1973;248:4092–4100. - PubMed
-
- Buckland PR, Rickards CR, Howells RD, Davies Jones E, Rees Smith B. Photo-affinity labelling of the thyrotropin receptor. FEBS Lett. 1982;145:245–249.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

