Exendin-4 promotes extracellular-superoxide dismutase expression in A549 cells through DNA demethylation
- PMID: 26798195
- PMCID: PMC4706090
- DOI: 10.3164/jcbn.15-16
Exendin-4 promotes extracellular-superoxide dismutase expression in A549 cells through DNA demethylation
Abstract
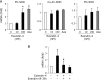
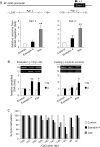
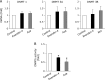
Exendin-4 is an agonist of the glucagon-like peptide 1 receptor (GLP-1R) and is used in the treatment of type 2 diabetes. Since human GLP-1R has been identified in various cells besides pancreatic cells, exendin-4 is expected to exert extrapancreatic actions. It has also been suggested to affect gene expression through epigenetic regulation, such as DNA methylation and/or histone modifications. Furthermore, the expression of extracellular-superoxide dismutase (EC-SOD), a major SOD isozyme that is crucially involved in redox homeostasis, is regulated by epigenetic factors. In the present study, we demonstrated that exendin-4 induced the demethylation of DNA in A549 cells, which, in turn, affected the expression of EC-SOD. Our results showed that the treatment with exendin-4 up-regulated the expression of EC-SOD through GLP-1R and demethylated some methyl-CpG sites (methylated cytosine at 5'-CG-3') in the EC-SOD gene. Moreover, the treatment with exendin-4 inactivated DNA methyltransferases (DNMTs), but did not change their expression levels. In conclusion, the results of the present study demonstrated for the first time that exendin-4 regulated the expression of EC-SOD by reducing the activity of DNMTs and demethylation of DNA within the EC-SOD promoter region in A549 cells.
Keywords: DNA demethylation; epigenetics; exendin-4; extracellular-superoxide dismutase.
Figures
Similar articles
-
Exendin-4 induces extracellular-superoxide dismutase through histone H3 acetylation in human retinal endothelial cells.J Clin Biochem Nutr. 2016 Nov;59(3):174-181. doi: 10.3164/jcbn.16-26. Epub 2016 Sep 8. J Clin Biochem Nutr. 2016. PMID: 27895384 Free PMC article.
-
Epigenetic regulation of extracellular-superoxide dismutase in human monocytes.Free Radic Biol Med. 2013 Aug;61:197-205. doi: 10.1016/j.freeradbiomed.2013.04.013. Epub 2013 Apr 18. Free Radic Biol Med. 2013. PMID: 23602908
-
CpG methylation attenuates Sp1 and Sp3 binding to the human extracellular superoxide dismutase promoter and regulates its cell-specific expression.Free Radic Biol Med. 2010 Apr 1;48(7):895-904. doi: 10.1016/j.freeradbiomed.2010.01.007. Epub 2010 Jan 14. Free Radic Biol Med. 2010. PMID: 20079429 Free PMC article.
-
Exendin-4 from Heloderma suspectum venom: From discovery to its latest application as type II diabetes combatant.Basic Clin Pharmacol Toxicol. 2019 May;124(5):513-527. doi: 10.1111/bcpt.13169. Epub 2018 Dec 12. Basic Clin Pharmacol Toxicol. 2019. PMID: 30417596 Review.
-
Mouse extracellular superoxide dismutase: primary structure, tissue-specific gene expression, chromosomal localization, and lung in situ hybridization.Am J Respir Cell Mol Biol. 1997 Oct;17(4):393-403. doi: 10.1165/ajrcmb.17.4.2826. Am J Respir Cell Mol Biol. 1997. PMID: 9376114 Review.
Cited by
-
Copper in the tumor microenvironment and tumor metastasis.J Clin Biochem Nutr. 2022 Jul;71(1):22-28. doi: 10.3164/jcbn.22-9. Epub 2022 May 13. J Clin Biochem Nutr. 2022. PMID: 35903604 Free PMC article.
-
Clinical relevance of epigenetics in the onset and management of type 2 diabetes mellitus.Epigenetics. 2017 Jun 3;12(6):401-415. doi: 10.1080/15592294.2016.1278097. Epub 2017 Jan 6. Epigenetics. 2017. PMID: 28059593 Free PMC article. Review.
-
Role of copper and SOD3-mediated extracellular redox regulation in tumor progression.J Clin Biochem Nutr. 2024 Jul;75(1):1-6. doi: 10.3164/jcbn.24-14. Epub 2024 Apr 6. J Clin Biochem Nutr. 2024. PMID: 39070539 Free PMC article.
-
Exendin-4 induces extracellular-superoxide dismutase through histone H3 acetylation in human retinal endothelial cells.J Clin Biochem Nutr. 2016 Nov;59(3):174-181. doi: 10.3164/jcbn.16-26. Epub 2016 Sep 8. J Clin Biochem Nutr. 2016. PMID: 27895384 Free PMC article.
-
Exendin-4 alleviates β-Amyloid peptide toxicity via DAF-16 in a Caenorhabditis elegans model of Alzheimer's disease.Front Aging Neurosci. 2022 Aug 5;14:955113. doi: 10.3389/fnagi.2022.955113. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35992601 Free PMC article.
References
-
- Cave AC, Brewer AC, Narayanapanicker A, et al. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal. 2006;8:691–728. - PubMed
-
- Vachier I, Chanez P, Le Doucen C, Damon M, Descomps B, Godard P. Enhancement of reactive oxygen species formation in stable and unstable asthmatic patients. Eur Respir J. 1994;7:1585–1592. - PubMed
-
- Ookawara T, Imazeki N, Matsubara O, et al. Tissue distribution of immunoreactive mouse extracellular superoxide dismutase. Am J Physiol. 1998;275 (3 Pt 1):C840–C847. - PubMed
-
- Folz RJ, Guan J, Seldin MF, Oury TD, Enghild JJ, Crapo JD. Mouse extracellular superoxide dismutase: primary structure, tissue-specific gene expression, chromosomal localization, and lung in situ hybridization. Am J Respir Cell Mol Biol. 1997;17:393–403. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

