Chemical tools for the study of hydrogen sulfide (H2S) and sulfane sulfur and their applications to biological studies
- PMID: 26798192
- PMCID: PMC4706096
- DOI: 10.3164/jcbn.15-91
Chemical tools for the study of hydrogen sulfide (H2S) and sulfane sulfur and their applications to biological studies
Abstract
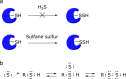
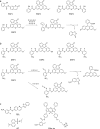
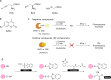
Hydrogen sulfide (H2S) functions in many physiological processes, including relaxation of vascular smooth muscles, mediation of neurotransmission, inhibition of insulin signaling, and regulation of inflammation. On the other hand, sulfane sulfur, which is a sulfur atom with six valence electrons but no charge, has the unique ability to bind reversibly to other sulfur atoms to form hydropersulfides (R-S-SH) and polysulfides (-S-Sn-S-). H2S and sulfane sulfur always coexist, and recent work suggests that sulfane sulfur species may be the actual signaling molecules in at least some biological phenomena. For example, one of the mechanisms of activity regulation of proteins by H2S is the S-sulfhydration of cysteine residues (protein Cys-SSH). In this review, we summarize recent progress on chemical tools for the study of H2S and sulfane sulfur, covering fluorescence probes utilizing various design strategies, H2S caged compounds, inhibitors of physiological H2S-producing enzymes (cystathionine γ-lyase, cystathionine β-synthase and 3-mercaptopyruvate sulfurtransferase), and labeling reagents. Fluorescence probes offer particular advantages as chemical tools to study physiological functions of biomolecules, including ease of use and real-time, nondestructive visualization of biological processes in live cells and tissues.
Keywords: caged compound; enzyme inhibitor; fluorescence probe; hydrogen sulfide; sulfane sulfur.
Figures
Similar articles
-
Fluorescent Probes and Selective Inhibitors for Biological Studies of Hydrogen Sulfide- and Polysulfide-Mediated Signaling.Antioxid Redox Signal. 2017 Oct 1;27(10):669-683. doi: 10.1089/ars.2017.7070. Epub 2017 May 18. Antioxid Redox Signal. 2017. PMID: 28443673 Free PMC article. Review.
-
Fluorescent probes for hydrogen sulfide (H2S) and sulfane sulfur and their applications to biological studies.Nitric Oxide. 2015 Apr 30;46:72-9. doi: 10.1016/j.niox.2014.11.008. Epub 2014 Nov 13. Nitric Oxide. 2015. PMID: 25461270 Review.
-
The physiological role of hydrogen sulfide and beyond.Nitric Oxide. 2014 Sep 15;41:4-10. doi: 10.1016/j.niox.2014.01.002. Epub 2014 Feb 1. Nitric Oxide. 2014. PMID: 24491257 Review.
-
Escherichia coli Uses Separate Enzymes to Produce H2S and Reactive Sulfane Sulfur From L-cysteine.Front Microbiol. 2019 Feb 20;10:298. doi: 10.3389/fmicb.2019.00298. eCollection 2019. Front Microbiol. 2019. PMID: 30873134 Free PMC article.
-
[Development of fluorescent probes for detecting reactive sulfur species and their application to development of inhibitors for 3MST].Nihon Yakurigaku Zasshi. 2019;154(3):121-127. doi: 10.1254/fpj.154.121. Nihon Yakurigaku Zasshi. 2019. PMID: 31527361 Japanese.
Cited by
-
HPLC Study of Product Formed in the Reaction of NBD-Derived Fluorescent Probe with Hydrogen Sulfide, Cysteine, N-acetylcysteine, and Glutathione.Molecules. 2022 Nov 28;27(23):8305. doi: 10.3390/molecules27238305. Molecules. 2022. PMID: 36500398 Free PMC article.
-
Desulfurization of thiosemicarbazones: the role of metal ions and biological implications.J Biol Inorg Chem. 2024 Feb;29(1):3-31. doi: 10.1007/s00775-023-02037-7. Epub 2023 Dec 26. J Biol Inorg Chem. 2024. PMID: 38148423 Review.
-
A selective and sensitive method for quantification of endogenous polysulfide production in biological samples.Redox Biol. 2018 Sep;18:295-304. doi: 10.1016/j.redox.2018.07.016. Epub 2018 Jul 21. Redox Biol. 2018. PMID: 30077923 Free PMC article.
-
Fluorescent Probes and Selective Inhibitors for Biological Studies of Hydrogen Sulfide- and Polysulfide-Mediated Signaling.Antioxid Redox Signal. 2017 Oct 1;27(10):669-683. doi: 10.1089/ars.2017.7070. Epub 2017 May 18. Antioxid Redox Signal. 2017. PMID: 28443673 Free PMC article. Review.
-
Vascular biology of hydrogen sulfide.Am J Physiol Cell Physiol. 2017 May 1;312(5):C537-C549. doi: 10.1152/ajpcell.00329.2016. Epub 2017 Feb 1. Am J Physiol Cell Physiol. 2017. PMID: 28148499 Free PMC article. Review.
References
-
- Paul BD, Snyder SH. H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol. 2012;13:499–507. - PubMed
-
- Kimura H. Physiological role of hydrogen sulfide and polysulfide in the central nervous system. Neurochem Int. 2013;63:492–497. - PubMed
-
- Kaneko Y, Kimura Y, Kimura H, Niki I. l-Cysteine inhibits insulin release from the pancreatic β-cell: possible involvement of metabolic production of hydrogen sulfide, a novel gasotransmitter. Diabetes. 2006;55:1391–1397. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

