CXCL13 promotes isotype-switched B cell accumulation to the central nervous system during viral encephalomyelitis
- PMID: 26795429
- PMCID: PMC4828287
- DOI: 10.1016/j.bbi.2016.01.016
CXCL13 promotes isotype-switched B cell accumulation to the central nervous system during viral encephalomyelitis
Abstract
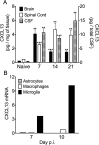
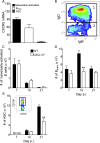
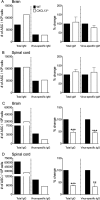
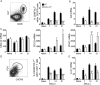
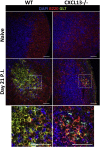
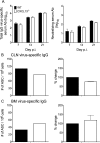
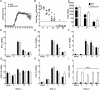
Elevated CXCL13 within the central nervous system (CNS) correlates with humoral responses in several neuroinflammatory diseases, yet its role is controversial. During coronavirus encephalomyelitis CXCL13 deficiency impaired CNS accumulation of memory B cells and antibody-secreting cells (ASC) but not naïve/early-activated B cells. However, despite diminished germinal center B cells and follicular helper T cells in draining lymph nodes, ASC in bone marrow and antiviral serum antibody were intact in the absence of CXCL13. The data demonstrate that CXCL13 is not essential in mounting effective peripheral humoral responses, but specifically promotes CNS accumulation of differentiated B cells.
Keywords: Antibody secreting cells; B cells; CXCL13; Central nervous system; Memory B cells; Neuroimmunology.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Activated GL7+ B cells are maintained within the inflamed CNS in the absence of follicle formation during viral encephalomyelitis.Brain Behav Immun. 2017 Feb;60:71-83. doi: 10.1016/j.bbi.2016.09.022. Epub 2016 Sep 19. Brain Behav Immun. 2017. PMID: 27658544 Free PMC article.
-
Protective Humoral Immunity in the Central Nervous System Requires Peripheral CD19-Dependent Germinal Center Formation following Coronavirus Encephalomyelitis.J Virol. 2017 Nov 14;91(23):e01352-17. doi: 10.1128/JVI.01352-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28931676 Free PMC article.
-
Progression from IgD+ IgM+ to isotype-switched B cells is site specific during coronavirus-induced encephalomyelitis.J Virol. 2014 Aug;88(16):8853-67. doi: 10.1128/JVI.00861-14. Epub 2014 May 28. J Virol. 2014. PMID: 24872583 Free PMC article.
-
Finding the right niche: B-cell migration in the early phases of T-dependent antibody responses.Int Immunol. 2010 Jun;22(6):413-9. doi: 10.1093/intimm/dxq047. Int Immunol. 2010. PMID: 20508253 Free PMC article. Review.
-
CXCR5(+) T cells: follicular homing takes center stage in T-helper-cell responses.Trends Immunol. 2002 May;23(5):250-4. doi: 10.1016/s1471-4906(02)02218-4. Trends Immunol. 2002. PMID: 12102746 Review. No abstract available.
Cited by
-
B Cells in Multiple Sclerosis and Virus-Induced Neuroinflammation.Front Neurol. 2020 Nov 3;11:591894. doi: 10.3389/fneur.2020.591894. eCollection 2020. Front Neurol. 2020. PMID: 33224101 Free PMC article. Review.
-
Activated GL7+ B cells are maintained within the inflamed CNS in the absence of follicle formation during viral encephalomyelitis.Brain Behav Immun. 2017 Feb;60:71-83. doi: 10.1016/j.bbi.2016.09.022. Epub 2016 Sep 19. Brain Behav Immun. 2017. PMID: 27658544 Free PMC article.
-
Asymptomatic sensitization to a cow's milk protein induces sustained neuroinflammation and behavioral changes with chronic allergen exposure.Front Allergy. 2022 Sep 7;3:870628. doi: 10.3389/falgy.2022.870628. eCollection 2022. Front Allergy. 2022. PMID: 36157272 Free PMC article.
-
The Brave New World of Early Treatment of Multiple Sclerosis: Using the Molecular Biomarkers CXCL13 and Neurofilament Light to Optimize Immunotherapy.Biomedicines. 2022 Aug 28;10(9):2099. doi: 10.3390/biomedicines10092099. Biomedicines. 2022. PMID: 36140203 Free PMC article. Review.
-
Central Nervous System Inflammatory Aggregates in the Theiler's Virus Model of Progressive Multiple Sclerosis.Front Immunol. 2019 Aug 2;10:1821. doi: 10.3389/fimmu.2019.01821. eCollection 2019. Front Immunol. 2019. PMID: 31428102 Free PMC article.
References
-
- Ansel K.M., Ngo V.N., Hyman P.L., Luther S.A., Forster R., Sedgwick J.D., Browning J.L., Lipp M., Cyster J.G. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. - PubMed
-
- Bagaeva L.V., Rao P., Powers J.M., Segal B.M. CXC chemokine ligand 13 plays a role in experimental autoimmune encephalomyelitis. J. Immunol. 2006;176:7676–7685. - PubMed
-
- Bergmann C.C., Altman J.D., Hinton D., Stohlman S.A. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 1999;163:3379–3387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

