The First Alcohol Drink Triggers mTORC1-Dependent Synaptic Plasticity in Nucleus Accumbens Dopamine D1 Receptor Neurons
- PMID: 26791202
- PMCID: PMC4719011
- DOI: 10.1523/JNEUROSCI.2254-15.2016
The First Alcohol Drink Triggers mTORC1-Dependent Synaptic Plasticity in Nucleus Accumbens Dopamine D1 Receptor Neurons
Erratum in
- J Neurosci. 2016 Apr 13;36(15):4400-3
-
Erratum: Beckley et al., "The First Alcohol Drink Triggers mTORC1-Dependent Synaptic Plasticity in Nucleus Accumbens Dopamine D1 Receptor Neurons".J Neurosci. 2022 Sep 21;42(38):7330. doi: 10.1523/JNEUROSCI.1404-22.2022. J Neurosci. 2022. PMID: 38557400 Free PMC article. No abstract available.
Abstract
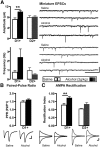
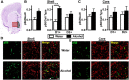
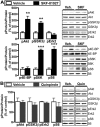
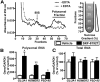
Early binge-like alcohol drinking may promote the development of hazardous intake. However, the enduring cellular alterations following the first experience with alcohol consumption are not fully understood. We found that the first binge-drinking alcohol session produced enduring enhancement of excitatory synaptic transmission onto dopamine D1 receptor-expressing neurons (D1+ neurons) in the nucleus accumbens (NAc) shell but not the core in mice, which required D1 receptors (D1Rs) and mechanistic target of rapamycin complex 1 (mTORC1). Furthermore, inhibition of mTORC1 activity during the first alcohol drinking session reduced alcohol consumption and preference of a subsequent drinking session. mTORC1 is critically involved in RNA-to-protein translation, and we found that the first alcohol session rapidly activated mTORC1 in NAc shell D1+ neurons and increased synaptic expression of the AMPAR subunit GluA1 and the scaffolding protein Homer. Finally, D1R stimulation alone was sufficient to activate mTORC1 in the NAc to promote mTORC1-dependent translation of the synaptic proteins GluA1 and Homer. Together, our results indicate that the first alcohol drinking session induces synaptic plasticity in NAc D1+ neurons via enhanced mTORC1-dependent translation of proteins involved in excitatory synaptic transmission that in turn drives the reinforcement learning associated with the first alcohol experience. Thus, the alcohol-dependent D1R/mTORC1-mediated increase in synaptic function in the NAc may reflect a neural imprint of alcohol's reinforcing properties, which could promote subsequent alcohol intake. Significance statement: Consuming alcohol for the first time is a learning event that drives further drinking. Here, we identified a mechanism that may underlie the reinforcing learning associated with the initial alcohol experience. We show that the first alcohol experience induces a persistent enhancement of excitatory synaptic transmission on NAc shell D1+ neurons, which is dependent on D1R and mTORC1. We also find that mTORC1 is necessary for the sustained alcohol consumption and preference across the initial drinking sessions. The first alcohol binge activates mTORC1 in NAc D1+ neurons and increases levels of synaptic proteins involved in glutamatergic signaling. Thus, the D1R/mTORC1-dependent plasticity following the first alcohol exposure may be a critical cellular component of reinforcement learning.
Keywords: addiction; alcohol; dopamine; mTOR; plasticity.
Copyright © 2016 the authors 0270-6474/16/360701-13$15.00/0.
Figures



Similar articles
-
Dopamine Receptors Differentially Control Binge Alcohol Drinking-Mediated Synaptic Plasticity of the Core Nucleus Accumbens Direct and Indirect Pathways.J Neurosci. 2017 May 31;37(22):5463-5474. doi: 10.1523/JNEUROSCI.3845-16.2017. Epub 2017 May 4. J Neurosci. 2017. PMID: 28473645 Free PMC article.
-
Selective alterations of NMDAR function and plasticity in D1 and D2 medium spiny neurons in the nucleus accumbens shell following chronic intermittent ethanol exposure.Neuropharmacology. 2017 Jan;112(Pt A):164-171. doi: 10.1016/j.neuropharm.2016.03.004. Epub 2016 Mar 2. Neuropharmacology. 2017. PMID: 26946430 Free PMC article.
-
Prosapip1-Dependent Synaptic Adaptations in the Nucleus Accumbens Drive Alcohol Intake, Seeking, and Reward.Neuron. 2017 Sep 27;96(1):145-159.e8. doi: 10.1016/j.neuron.2017.08.037. Epub 2017 Sep 8. Neuron. 2017. PMID: 28890345 Free PMC article.
-
Regulation of AMPA receptor trafficking in the nucleus accumbens by dopamine and cocaine.Neurotox Res. 2010 Nov;18(3-4):393-409. doi: 10.1007/s12640-010-9176-0. Epub 2010 Apr 2. Neurotox Res. 2010. PMID: 20361291 Free PMC article. Review.
-
Mechanisms by which dopamine receptors may influence synaptic plasticity.Ann N Y Acad Sci. 2003 Nov;1003:241-9. doi: 10.1196/annals.1300.015. Ann N Y Acad Sci. 2003. PMID: 14684450 Review.
Cited by
-
Synaptic Effects Induced by Alcohol.Curr Top Behav Neurosci. 2023 Feb 11:10.1007/7854_2022_412. doi: 10.1007/7854_2022_412. Online ahead of print. Curr Top Behav Neurosci. 2023. PMID: 36765015 Free PMC article.
-
Alcohol Dependence Modulates Amygdalar mTORC2 and PKCε Expression in a Rodent Model.Nutrients. 2023 Jul 5;15(13):3036. doi: 10.3390/nu15133036. Nutrients. 2023. PMID: 37447362 Free PMC article.
-
GluN2B Subunit of the NMDA Receptor: The Keystone of the Effects of Alcohol During Neurodevelopment.Neurochem Res. 2019 Jan;44(1):78-88. doi: 10.1007/s11064-017-2462-y. Epub 2018 Jan 6. Neurochem Res. 2019. PMID: 29307084 Review.
-
Protein Translation in the Nucleus Accumbens Is Dysregulated during Cocaine Withdrawal and Required for Expression of Incubation of Cocaine Craving.J Neurosci. 2018 Mar 14;38(11):2683-2697. doi: 10.1523/JNEUROSCI.2412-17.2018. Epub 2018 Feb 5. J Neurosci. 2018. PMID: 29431650 Free PMC article.
-
Alcohol-dependent molecular adaptations of the NMDA receptor system.Genes Brain Behav. 2017 Jan;16(1):139-148. doi: 10.1111/gbb.12363. Genes Brain Behav. 2017. PMID: 27906494 Free PMC article. Review.
References
-
- Ben Hamida S, Neasta J, Lasek AW, Kharazia V, Zou M, Carnicella S, Janak PH, Ron D. The small G protein H-Ras in the mesolimbic system is a molecular gateway to alcohol-seeking and excessive drinking behaviors. J Neurosci. 2012;32:15849–15858. doi: 10.1523/JNEUROSCI.2846-12.2012. - DOI - PMC - PubMed
-
- Biever A, Puighermanal E, Nishi A, David A, Panciatici C, Longueville S, Xirodimas D, Gangarossa G, Meyuhas O, Hervé D, Girault JA, Valjent E. PKA-dependent phosphorylation of ribosomal protein S6 does not correlate with translation efficiency in striatonigral and striatopallidal medium-sized spiny neurons. J Neurosci. 2015;35:4113–4130. doi: 10.1523/JNEUROSCI.3288-14.2015. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
