Protein purification and cloning of diacylglycerol lipase from rat brain
- PMID: 26790472
- PMCID: PMC4892392
- DOI: 10.1093/jb/mvw002
Protein purification and cloning of diacylglycerol lipase from rat brain
Abstract
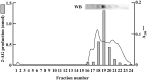
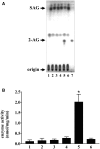
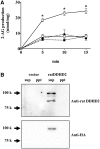
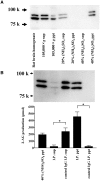
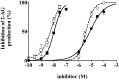
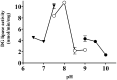
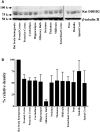
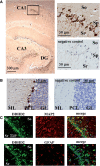
Diacylglycerol (DG) lipase, which hydrolyses 1-stearoyl-2-arachidonyl-sn-glycerol to produce an endocannabinoid, 2-arachidonoylglycerol, was purified from the soluble fraction of rat brain lysates. DG lipase was purified about 1,200-fold by a sequential column chromatographic procedure. Among proteins identified by mass spectrometry analysis in the partially purified DG lipase sample, only DDHD domain containing two (DDHD2), which was formerly regarded as a phospholipase A1, exhibited significant DG lipase activity. Rat DDHD2 expressed in Chinese hamster ovary cells showed similar enzymatic properties to partially purified DG lipase from rat brain. The source of DG lipase activity in rat brain was immunoprecipitated using anti-DDHD2 antibody. Thus, we concluded that the DG lipase activity in the soluble fraction of rat brain is derived from DDHD2. DDHD2 is distributed widely in the rat brain. Immunohistochemical analysis revealed that DDHD2 is expressed in hippocampal neurons, but not in glia.
Keywords: 2-arachidonoylglycerol; diacylglycerol; endocannabinoid; lipase; phospholipase.
© The Authors 2016. Published by Oxford University Press on behalf of the Japanese Biochemical Society. All rights reserved.
Figures


Similar articles
-
Enzymatic characterization of recombinant rat DDHD2: a soluble diacylglycerol lipase.J Biochem. 2016 Nov;160(5):269-279. doi: 10.1093/jb/mvw034. Epub 2016 May 19. J Biochem. 2016. PMID: 27198176
-
Brain regional cannabinoid CB(1) receptor signalling and alternative enzymatic pathways for 2-arachidonoylglycerol generation in brain sections of diacylglycerol lipase deficient mice.Eur J Pharm Sci. 2014 Jan 23;51:87-95. doi: 10.1016/j.ejps.2013.08.035. Epub 2013 Sep 3. Eur J Pharm Sci. 2014. PMID: 24012970
-
Biosynthesis and degradation of the endocannabinoid 2-arachidonoylglycerol.Biofactors. 2011 Jan-Feb;37(1):1-7. doi: 10.1002/biof.131. Epub 2010 Nov 29. Biofactors. 2011. PMID: 21328621 Review.
-
Age-related changes in the endocannabinoid system in the mouse hippocampus.Mech Ageing Dev. 2015 Sep;150:55-64. doi: 10.1016/j.mad.2015.08.005. Epub 2015 Aug 13. Mech Ageing Dev. 2015. PMID: 26278494
-
Regulation of adult neurogenesis by the endocannabinoid-producing enzyme diacylglycerol lipase alpha (DAGLa).Sci Rep. 2022 Jan 12;12(1):633. doi: 10.1038/s41598-021-04600-1. Sci Rep. 2022. PMID: 35022487 Free PMC article.
Cited by
-
Lipolysis: cellular mechanisms for lipid mobilization from fat stores.Nat Metab. 2021 Nov;3(11):1445-1465. doi: 10.1038/s42255-021-00493-6. Epub 2021 Nov 19. Nat Metab. 2021. PMID: 34799702 Review.
-
DDHD1, but Not DDHD2, Suppresses Neurite Outgrowth in SH-SY5Y and PC12 Cells by Regulating Protein Transport From Recycling Endosomes.Front Cell Dev Biol. 2020 Jul 23;8:670. doi: 10.3389/fcell.2020.00670. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32850804 Free PMC article.
-
From Classical to Alternative Pathways of 2-Arachidonoylglycerol Synthesis: AlterAGs at the Crossroad of Endocannabinoid and Lysophospholipid Signaling.Molecules. 2024 Aug 4;29(15):3694. doi: 10.3390/molecules29153694. Molecules. 2024. PMID: 39125098 Free PMC article. Review.
-
Lipid-metabolizing serine hydrolases in the mammalian central nervous system: endocannabinoids and beyond.Biochim Biophys Acta Mol Cell Biol Lipids. 2019 Jun;1864(6):907-921. doi: 10.1016/j.bbalip.2018.08.007. Epub 2018 Aug 16. Biochim Biophys Acta Mol Cell Biol Lipids. 2019. PMID: 30905349 Free PMC article. Review.
-
Loss of DDHD2, whose mutation causes spastic paraplegia, promotes reactive oxygen species generation and apoptosis.Cell Death Dis. 2018 Jul 23;9(8):797. doi: 10.1038/s41419-018-0815-3. Cell Death Dis. 2018. PMID: 30038238 Free PMC article.
References
-
- Wang J., Ueda N. (2008) Role of the endocannabinoid system in metabolic control. Curr. Opin. Nephrol. Hypertens. 17, 1–10 - PubMed
-
- Sugiura T., Kishimoto S., Oka S., Gokoh M. (2006) Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog. Lipid Res. 45, 405–446 - PubMed
-
- Matsuda L.A., Bonner T.I., Lolait S.J. (1993) Localization of cannabinoid receptor mRNA in rat brain. J. Comp. Neurol. 327, 535–550 - PubMed
-
- Tsou K., Brown S., Sanudo-Pena M.C., Mackie K., Walker J.M. (1998) Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83, 393–411 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

