Synaptic circuitry of identified neurons in the antennal lobe of Drosophila melanogaster
- PMID: 26780543
- PMCID: PMC6680330
- DOI: 10.1002/cne.23966
Synaptic circuitry of identified neurons in the antennal lobe of Drosophila melanogaster
Abstract
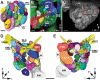
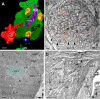
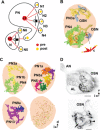
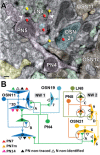
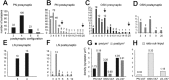
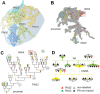
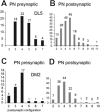
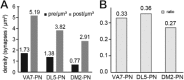
In Drosophila melanogaster olfactory sensory neurons (OSNs) establish synapses with projection neurons (PNs) and local interneurons within antennal lobe (AL) glomeruli. Substantial knowledge regarding this circuitry has been obtained by functional studies, whereas ultrastructural evidence of synaptic contacts is scarce. To fill this gap, we studied serial sections of three glomeruli using electron microscopy. Ectopic expression of a membrane-bound peroxidase allowed us to map synaptic sites along PN dendrites. Our data prove for the first time that each of the three major types of AL neurons is both pre- and postsynaptic to the other two types, as previously indicated by functional studies. PN dendrites carry a large proportion of output synapses, with approximately one output per every three input synapses. Detailed reconstructions of PN dendrites showed that these synapses are distributed unevenly, with input and output sites partially segregated along a proximal-distal gradient and the thinnest branches carrying solely input synapses. Moreover, our data indicate synapse clustering, as we found evidence of dendritic tiling of PN dendrites. PN output synapses exhibited T-shaped presynaptic densities, mostly arranged as tetrads. In contrast, output synapses from putative OSNs showed elongated presynaptic densities in which the T-bar platform was supported by several pedestals and contacted as many as 20 postsynaptic profiles. We also discovered synaptic contacts between the putative OSNs. The average synaptic density in the glomerular neuropil was about two synapses/µm(3) . These results are discussed with regard to current models of olfactory glomerular microcircuits across species.
Keywords: Drosophila melanogaster; glomerulus; olfactory system; projection neuron; synaptic microcircuits; ultrastructure.
© 2016 The Authors The Journal of Comparative Neurology Published by Wiley Periodicals, Inc.
Conflict of interest statement
No authors have any known or potential conflict of interest including any financial, personal, or other relationships with other people or organizations within the years of beginning the submitted work that could inappropriately influence or be perceived to influence the work.
Figures





Similar articles
-
Different classes of input and output neurons reveal new features in microglomeruli of the adult Drosophila mushroom body calyx.J Comp Neurol. 2012 Jul 1;520(10):2185-201. doi: 10.1002/cne.23037. J Comp Neurol. 2012. PMID: 22237598
-
Synaptic connections of cholinergic antennal lobe relay neurons innervating the lateral horn neuropile in the brain of Drosophila melanogaster.J Comp Neurol. 2003 Nov 17;466(3):299-315. doi: 10.1002/cne.10867. J Comp Neurol. 2003. PMID: 14556288
-
Synaptic organization of the mushroom body calyx in Drosophila melanogaster.J Comp Neurol. 2002 Apr 8;445(3):211-26. doi: 10.1002/cne.10155. J Comp Neurol. 2002. PMID: 11920702
-
Development of olfactory projection neuron dendrites that contribute to wiring specificity of the Drosophila olfactory circuit.Genes Genet Syst. 2014;89(1):17-26. doi: 10.1266/ggs.89.17. Genes Genet Syst. 2014. PMID: 24817758 Review.
-
Developmental experience-dependent plasticity in the first synapse of the Drosophila olfactory circuit.J Neurophysiol. 2016 Dec 1;116(6):2730-2738. doi: 10.1152/jn.00616.2016. Epub 2016 Sep 28. J Neurophysiol. 2016. PMID: 27683892 Free PMC article. Review.
Cited by
-
The wiring diagram of a glomerular olfactory system.Elife. 2016 May 13;5:e14859. doi: 10.7554/eLife.14859. Elife. 2016. PMID: 27177418 Free PMC article.
-
A resource for the Drosophila antennal lobe provided by the connectome of glomerulus VA1v.Elife. 2018 Nov 1;7:e37550. doi: 10.7554/eLife.37550. Elife. 2018. PMID: 30382940 Free PMC article.
-
Sexual dimorphism in visual and olfactory brain centers in the perfume-collecting orchid bee Euglossa dilemma (Hymenoptera, Apidae).J Comp Neurol. 2018 Sep 1;526(13):2068-2077. doi: 10.1002/cne.24483. Epub 2018 Aug 8. J Comp Neurol. 2018. PMID: 30088672 Free PMC article.
-
Experience-dependent plasticity in the olfactory system of Drosophila melanogaster and other insects.Front Cell Neurosci. 2023 Feb 22;17:1130091. doi: 10.3389/fncel.2023.1130091. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36923450 Free PMC article. Review.
-
Chronic exposure to odors at naturally occurring concentrations triggers limited plasticity in early stages of Drosophila olfactory processing.Elife. 2023 May 30;12:e85443. doi: 10.7554/eLife.85443. Elife. 2023. PMID: 37195027 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

