The Neutrophil Btk Signalosome Regulates Integrin Activation during Sterile Inflammation
- PMID: 26777396
- PMCID: PMC5030078
- DOI: 10.1016/j.immuni.2015.11.011
The Neutrophil Btk Signalosome Regulates Integrin Activation during Sterile Inflammation
Abstract
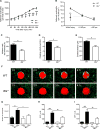
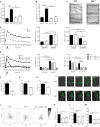
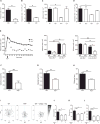
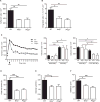
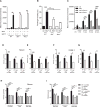
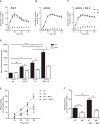
Neutrophils are recruited from the blood to sites of sterile inflammation, where they are involved in wound healing but can also cause tissue damage. During sterile inflammation, necrotic cells release pro-inflammatory molecules including formylated peptides. However, the signaling pathway triggered by formylated peptides to integrin activation and leukocyte recruitment is unknown. By using spinning-disk confocal intravital microscopy, we examined the molecular mechanisms of leukocyte recruitment to sites of focal hepatic necrosis in vivo. We demonstrated that the Bruton's tyrosine kinase (Btk) was required for multiple Mac-1 activation events involved in neutrophil recruitment and functions during sterile inflammation triggered by fMLF. The Src family kinase Hck, Wiskott-Aldrich-syndrome protein, and phospholipase Cγ2 were also involved in this pathway required for fMLF-triggered Mac-1 activation and neutrophil recruitment. Thus, we have identified a neutrophil Btk signalosome that is involved in a signaling pathway triggered by formylated peptides leading to the selective activation of Mac-1 and neutrophil recruitment during sterile inflammation.
Keywords: Btk; Src family kinases; WASp; fMLF; integrin; phospholipase C; signaling.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
PRN473, an inhibitor of Bruton's tyrosine kinase, inhibits neutrophil recruitment via inhibition of macrophage antigen-1 signalling.Br J Pharmacol. 2018 Feb;175(3):429-439. doi: 10.1111/bph.14090. Epub 2017 Dec 22. Br J Pharmacol. 2018. PMID: 29130484 Free PMC article.
-
The role of the tec kinase Bruton's tyrosine kinase (Btk) in leukocyte recruitment.Int Rev Immunol. 2012 Apr;31(2):104-18. doi: 10.3109/08830185.2012.668982. Int Rev Immunol. 2012. PMID: 22449072 Review.
-
Selective down-regulation of neutrophil Mac-1 in endotoxemic hepatic microcirculation via IL-10.J Immunol. 2009 Dec 1;183(11):7557-68. doi: 10.4049/jimmunol.0901786. Epub 2009 Nov 16. J Immunol. 2009. PMID: 19917697
-
Intravascular danger signals guide neutrophils to sites of sterile inflammation.Science. 2010 Oct 15;330(6002):362-6. doi: 10.1126/science.1195491. Science. 2010. PMID: 20947763
-
Neutrophil activation via beta2 integrins (CD11/CD18): molecular mechanisms and clinical implications.Thromb Haemost. 2007 Aug;98(2):262-73. Thromb Haemost. 2007. PMID: 17721605 Review.
Cited by
-
Current Perspectives: Evidence to Date on BTK Inhibitors in the Management of Multiple Sclerosis.Drug Des Devel Ther. 2022 Oct 6;16:3473-3490. doi: 10.2147/DDDT.S348129. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 36238195 Free PMC article. Review.
-
Bruton's tyrosine kinase inhibition attenuates disease progression by reducing renal immune cell invasion in mice with hemolytic-uremic syndrome.Front Immunol. 2023 Feb 23;14:1105181. doi: 10.3389/fimmu.2023.1105181. eCollection 2023. Front Immunol. 2023. PMID: 36911665 Free PMC article.
-
The Inhibition of Bruton Tyrosine Kinase Alleviates Acute Liver Failure via Downregulation of NLRP3 Inflammasome.J Immunol. 2022 Sep 15;209(6):1156-1164. doi: 10.4049/jimmunol.2001323. Epub 2022 Aug 17. J Immunol. 2022. PMID: 35977799 Free PMC article.
-
A Multi-Modal Toolkit for Studying Neutrophils in Cancer and Beyond.Cancers (Basel). 2021 Oct 23;13(21):5331. doi: 10.3390/cancers13215331. Cancers (Basel). 2021. PMID: 34771495 Free PMC article. Review.
-
Association between the AKT1 single nucleotide polymorphism (rs2498786, rs2494752 and rs5811155) and microscopic polyangiitis risk in a Chinese population.Mol Genet Genomics. 2023 May;298(3):767-776. doi: 10.1007/s00438-023-02012-6. Epub 2023 Apr 7. Mol Genet Genomics. 2023. PMID: 37029297 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

