Oncogenic CARMA1 couples NF-κB and β-catenin signaling in diffuse large B-cell lymphomas
- PMID: 26776161
- PMCID: PMC4981874
- DOI: 10.1038/onc.2015.493
Oncogenic CARMA1 couples NF-κB and β-catenin signaling in diffuse large B-cell lymphomas
Abstract
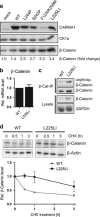
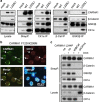
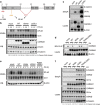
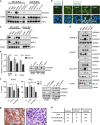
Constitutive activation of the antiapoptotic nuclear factor-κB (NF-κB) signaling pathway is a hallmark of the activated B-cell-like (ABC) subtype of diffuse large B-cell lymphomas (DLBCL). Recurrent oncogenic mutations are found in the scaffold protein CARMA1 (CARD11) that connects B-cell receptor (BCR) signaling to the canonical NF-κB pathway. We asked how far additional downstream processes are activated and contribute to the oncogenic potential of DLBCL-derived CARMA1 mutants. To this end, we expressed oncogenic CARMA1 in the NF-κB negative DLBCL lymphoma cell line BJAB. By a proteomic approach we identified recruitment of β-catenin and its destruction complex consisting of APC, AXIN1, CK1α and GSK3β to oncogenic CARMA1. Recruitment of the β-catenin destruction complex was independent of CARMA1-BCL10-MALT1 complex formation or constitutive NF-κB activation and promoted the stabilization of β-catenin. The β-catenin destruction complex was also recruited to CARMA1 in ABC DLBCL cell lines, which coincided with elevated β-catenin expression. In line, β-catenin was frequently detected in non-GCB DLBCL biopsies that rely on chronic BCR signaling. Increased β-catenin amounts alone were not sufficient to induce classical WNT target gene signatures, but could augment TCF/LEF-dependent transcriptional activation in response to WNT signaling. In conjunction with NF-κB, β-catenin enhanced expression of immunosuppressive interleukin-10 and suppressed antitumoral CCL3, indicating that β-catenin can induce a favorable tumor microenvironment. Thus, parallel activation of NF-κB and β-catenin signaling by gain-of-function mutations in CARMA1 augments WNT stimulation and is required for regulating the expression of distinct NF-κB target genes to trigger cell-intrinsic and extrinsic processes that promote DLBCL lymphomagenesis.
Figures

Similar articles
-
Lymphomagenic CARD11/BCL10/MALT1 signaling drives malignant B-cell proliferation via cooperative NF-κB and JNK activation.Proc Natl Acad Sci U S A. 2015 Dec 29;112(52):E7230-8. doi: 10.1073/pnas.1507459112. Epub 2015 Dec 14. Proc Natl Acad Sci U S A. 2015. PMID: 26668357 Free PMC article.
-
Combinatorial BTK and MALT1 inhibition augments killing of CD79 mutant diffuse large B cell lymphoma.Oncotarget. 2015 Dec 8;6(39):42232-42. doi: 10.18632/oncotarget.6273. Oncotarget. 2015. PMID: 26540570 Free PMC article.
-
Trimeric G protein-CARMA1 axis links smoothened, the hedgehog receptor transducer, to NF-κB activation in diffuse large B-cell lymphoma.Blood. 2013 Jun 6;121(23):4718-28. doi: 10.1182/blood-2012-12-470153. Epub 2013 Apr 30. Blood. 2013. PMID: 23632891 Free PMC article.
-
Role of the CARMA1/BCL10/MALT1 complex in lymphoid malignancies.Curr Opin Hematol. 2016 Jul;23(4):402-9. doi: 10.1097/MOH.0000000000000257. Curr Opin Hematol. 2016. PMID: 27135977 Free PMC article. Review.
-
Molecular pathways: targeting MALT1 paracaspase activity in lymphoma.Clin Cancer Res. 2013 Dec 15;19(24):6662-8. doi: 10.1158/1078-0432.CCR-12-3869. Epub 2013 Sep 4. Clin Cancer Res. 2013. PMID: 24004675 Review.
Cited by
-
Transducin β-like protein 1 controls multiple oncogenic networks in diffuse large B-cell lymphoma.Haematologica. 2021 Nov 1;106(11):2927-2939. doi: 10.3324/haematol.2020.268235. Haematologica. 2021. PMID: 33054136 Free PMC article.
-
Identification of B-cell translocation gene 1-controlled gene networks in diffuse large B-cell lymphoma: A study based on bioinformatics analysis.Oncol Lett. 2019 Mar;17(3):2825-2835. doi: 10.3892/ol.2019.9900. Epub 2019 Jan 8. Oncol Lett. 2019. PMID: 30854058 Free PMC article.
-
Large-Scale Proteomic Analysis of Follicular Lymphoma Reveals Extensive Remodeling of Cell Adhesion Pathway and Identifies Hub Proteins Related to the Lymphomagenesis.Cancers (Basel). 2021 Feb 5;13(4):630. doi: 10.3390/cancers13040630. Cancers (Basel). 2021. PMID: 33562532 Free PMC article.
-
CTD-2020K17.1, a Novel Long Non-Coding RNA, Promotes Migration, Invasion, and Proliferation of Serous Ovarian Cancer Cells In Vitro.Med Sci Monit. 2018 Mar 5;24:1329-1339. doi: 10.12659/msm.908456. Med Sci Monit. 2018. PMID: 29504606 Free PMC article.
-
Characterization of GECPAR, a noncoding RNA that regulates the transcriptional program of diffuse large B-cell lymphoma.Haematologica. 2022 May 1;107(5):1131-1143. doi: 10.3324/haematol.2020.267096. Haematologica. 2022. PMID: 34162177 Free PMC article.
References
-
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000; 403: 503–511. - PubMed
-
- Ngo VN, Davis RE, Lamy L, Yu X, Zhao H, Lenz G et al. A loss-of-function RNA interference screen for molecular targets in cancer. Nature 2006; 441: 106–110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

