Misassembly of full-length Disrupted-in-Schizophrenia 1 protein is linked to altered dopamine homeostasis and behavioral deficits
- PMID: 26754951
- PMCID: PMC5078859
- DOI: 10.1038/mp.2015.194
Misassembly of full-length Disrupted-in-Schizophrenia 1 protein is linked to altered dopamine homeostasis and behavioral deficits
Abstract
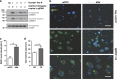
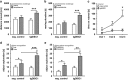
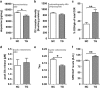
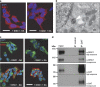
Disrupted-in-schizophrenia 1 (DISC1) is a mental illness gene first identified in a Scottish pedigree. So far, DISC1-dependent phenotypes in animal models have been confined to expressing mutant DISC1. Here we investigated how pathology of full-length DISC1 protein could be a major mechanism in sporadic mental illness. We demonstrate that a novel transgenic rat model, modestly overexpressing the full-length DISC1 transgene, showed phenotypes consistent with a significant role of DISC1 misassembly in mental illness. The tgDISC1 rat displayed mainly perinuclear DISC1 aggregates in neurons. Furthermore, the tgDISC1 rat showed a robust signature of behavioral phenotypes that includes amphetamine supersensitivity, hyperexploratory behavior and rotarod deficits, all pointing to changes in dopamine (DA) neurotransmission. To understand the etiology of the behavioral deficits, we undertook a series of molecular studies in the dorsal striatum of tgDISC1 rats. We observed an 80% increase in high-affinity DA D2 receptors, an increased translocation of the dopamine transporter to the plasma membrane and a corresponding increase in DA inflow as observed by cyclic voltammetry. A reciprocal relationship between DISC1 protein assembly and DA homeostasis was corroborated by in vitro studies. Elevated cytosolic dopamine caused an increase in DISC1 multimerization, insolubility and complexing with the dopamine transporter, suggesting a physiological mechanism linking DISC1 assembly and dopamine homeostasis. DISC1 protein pathology and its interaction with dopamine homeostasis is a novel cellular mechanism that is relevant for behavioral control and may have a role in mental illness.
Figures

Similar articles
-
Intra-nasal dopamine alleviates cognitive deficits in tgDISC1 rats which overexpress the human DISC1 gene.Neurobiol Learn Mem. 2017 Dec;146:12-20. doi: 10.1016/j.nlm.2017.10.015. Epub 2017 Oct 28. Neurobiol Learn Mem. 2017. PMID: 29107702
-
Disrupted-in-schizophrenia 1 Protein Misassembly Impairs Cognitive Flexibility and Social Behaviors in a Transgenic Rat Model.Neuroscience. 2022 Jun 15;493:41-51. doi: 10.1016/j.neuroscience.2022.04.013. Epub 2022 Apr 21. Neuroscience. 2022. PMID: 35461978
-
Enhanced dopamine function in DISC1-L100P mutant mice: implications for schizophrenia.Genes Brain Behav. 2010 Oct;9(7):777-89. doi: 10.1111/j.1601-183X.2010.00615.x. Epub 2010 Aug 12. Genes Brain Behav. 2010. PMID: 20618446
-
The impact of Disrupted-in-Schizophrenia 1 (DISC1) on the dopaminergic system: a systematic review.Transl Psychiatry. 2017 Jan 31;7(1):e1015. doi: 10.1038/tp.2016.282. Transl Psychiatry. 2017. PMID: 28140405 Free PMC article. Review.
-
Schizophrenia in translation: disrupted in schizophrenia (DISC1): integrating clinical and basic findings.Schizophr Bull. 2007 Jan;33(1):11-5. doi: 10.1093/schbul/sbl063. Epub 2006 Nov 30. Schizophr Bull. 2007. PMID: 17138582 Free PMC article. Review.
Cited by
-
DISC1 is a coordinator of intracellular trafficking to shape neuronal development and connectivity.J Physiol. 2016 Oct 1;594(19):5459-69. doi: 10.1113/JP272187. Epub 2016 Jun 12. J Physiol. 2016. PMID: 27121900 Free PMC article. Review.
-
Chronic N-Acetylcysteine Treatment Prevents Amphetamine-Induced Hyperactivity in Heterozygous Disc1 Mutant Mice, a Putative Prodromal Schizophrenia Animal Model.Int J Mol Sci. 2022 Aug 20;23(16):9419. doi: 10.3390/ijms23169419. Int J Mol Sci. 2022. PMID: 36012679 Free PMC article.
-
Thiol-mediated and catecholamine-enhanced multimerization of a cerebrovascular disease enriched fragment of NOTCH3.Exp Neurol. 2020 Jun;328:113261. doi: 10.1016/j.expneurol.2020.113261. Epub 2020 Feb 28. Exp Neurol. 2020. PMID: 32119934 Free PMC article.
-
Mechanisms underlying the role of DISC1 in synaptic plasticity.J Physiol. 2018 Jul;596(14):2747-2771. doi: 10.1113/JP274330. J Physiol. 2018. PMID: 30008190 Free PMC article. Review.
-
Disrupted-in-Schizophrenia 1 (DISC1) Overexpression and Juvenile Immune Activation Cause Sex-Specific Schizophrenia-Related Psychopathology in Rats.Front Psychiatry. 2019 Apr 9;10:222. doi: 10.3389/fpsyt.2019.00222. eCollection 2019. Front Psychiatry. 2019. PMID: 31057438 Free PMC article.
References
-
- Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet 2000; 9: 1415–1423. - PubMed
-
- St Clair D, Blackwood D, Muir W, Carothers A, Walker M, Spowart G et al. Association within a family of a balanced autosomal translocation with major mental illness. Lancet 1990; 336: 13–16. - PubMed
-
- Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Mol Psychiatry 2008; 13: 36–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

