Nesprin-2G, a Component of the Nuclear LINC Complex, Is Subject to Myosin-Dependent Tension
- PMID: 26745407
- PMCID: PMC4805861
- DOI: 10.1016/j.bpj.2015.11.014
Nesprin-2G, a Component of the Nuclear LINC Complex, Is Subject to Myosin-Dependent Tension
Abstract
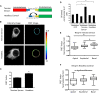
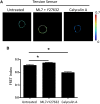
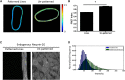
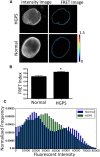
The nucleus of a cell has long been considered to be subject to mechanical force. Despite the observation that mechanical forces affect nuclear geometry and movement, how forces are applied onto the nucleus is not well understood. The nuclear LINC (linker of nucleoskeleton and cytoskeleton) complex has been hypothesized to be the critical structure that mediates the transfer of mechanical forces from the cytoskeleton onto the nucleus. Previously used techniques for studying nuclear forces have been unable to resolve forces across individual proteins, making it difficult to clearly establish if the LINC complex experiences mechanical load. To directly measure forces across the LINC complex, we generated a fluorescence resonance energy transfer-based tension biosensor for nesprin-2G, a key structural protein in the LINC complex, which physically links this complex to the actin cytoskeleton. Using this sensor we show that nesprin-2G is subject to mechanical tension in adherent fibroblasts, with highest levels of force on the apical and equatorial planes of the nucleus. We also show that the forces across nesprin-2G are dependent on actomyosin contractility and cell elongation. Additionally, nesprin-2G tension is reduced in fibroblasts from Hutchinson-Gilford progeria syndrome patients. This report provides the first, to our knowledge, direct evidence that nesprin-2G, and by extension the LINC complex, is subject to mechanical force. We also present evidence that nesprin-2G localization to the nuclear membrane is altered under high-force conditions. Because forces across the LINC complex are altered by a variety of different conditions, mechanical forces across the LINC complex, as well as the nucleus in general, may represent an important mechanism for mediating mechanotransduction.
Copyright © 2016 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
A Protocol for Using Förster Resonance Energy Transfer (FRET)-force Biosensors to Measure Mechanical Forces across the Nuclear LINC Complex.J Vis Exp. 2017 Apr 11;(122):54902. doi: 10.3791/54902. J Vis Exp. 2017. PMID: 28448008 Free PMC article.
-
Reinforcing the LINC complex connection to actin filaments: the role of FHOD1 in TAN line formation and nuclear movement.Cell Cycle. 2015;14(14):2200-5. doi: 10.1080/15384101.2015.1053665. Epub 2015 Jun 17. Cell Cycle. 2015. PMID: 26083340 Free PMC article.
-
Linker of nucleoskeleton and cytoskeleton (LINC) complex-mediated actin-dependent nuclear positioning orients centrosomes in migrating myoblasts.Nucleus. 2015;6(1):77-88. doi: 10.1080/19491034.2015.1004947. Nucleus. 2015. PMID: 25587885 Free PMC article.
-
Nesprin-3: a versatile connector between the nucleus and the cytoskeleton.Biochem Soc Trans. 2011 Dec;39(6):1719-24. doi: 10.1042/BST20110669. Biochem Soc Trans. 2011. PMID: 22103514 Review.
-
FHODs: Nuclear tethered formins for nuclear mechanotransduction.Front Cell Dev Biol. 2023 May 4;11:1160219. doi: 10.3389/fcell.2023.1160219. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37215084 Free PMC article. Review.
Cited by
-
Mechanical principles of nuclear shaping and positioning.J Cell Biol. 2018 Oct 1;217(10):3330-3342. doi: 10.1083/jcb.201804052. Epub 2018 Sep 7. J Cell Biol. 2018. PMID: 30194270 Free PMC article. Review.
-
The Desmosomal Cadherin Desmoglein-2 Experiences Mechanical Tension as Demonstrated by a FRET-Based Tension Biosensor Expressed in Living Cells.Cells. 2018 Jun 26;7(7):66. doi: 10.3390/cells7070066. Cells. 2018. PMID: 29949915 Free PMC article.
-
Nuclear lamina strain states revealed by intermolecular force biosensor.Nat Commun. 2023 Jun 30;14(1):3867. doi: 10.1038/s41467-023-39563-6. Nat Commun. 2023. PMID: 37391402 Free PMC article.
-
Inhibition of PDIs Downregulates Core LINC Complex Proteins, Promoting the Invasiveness of MDA-MB-231 Breast Cancer Cells in Confined Spaces In Vitro.Cells. 2024 May 24;13(11):906. doi: 10.3390/cells13110906. Cells. 2024. PMID: 38891038 Free PMC article.
-
Intranuclear Actin Structure Modulates Mesenchymal Stem Cell Differentiation.Stem Cells. 2017 Jun;35(6):1624-1635. doi: 10.1002/stem.2617. Epub 2017 Apr 3. Stem Cells. 2017. PMID: 28371128 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

