Ubiquitin phosphorylation in Parkinson's disease: Implications for pathogenesis and treatment
- PMID: 26740872
- PMCID: PMC4702311
- DOI: 10.1186/s40035-015-0049-6
Ubiquitin phosphorylation in Parkinson's disease: Implications for pathogenesis and treatment
Abstract
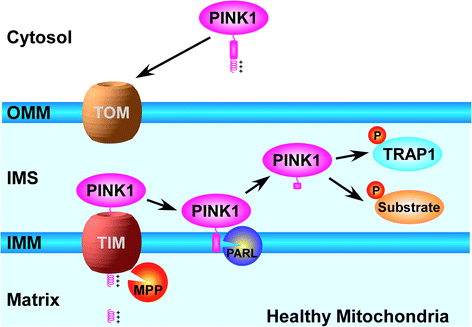
Parkinson's disease (PD) is the most common neurodegenerative movement disorder, characterized primarily by the loss of dopaminergic neurons in substantia nigra. The pathogenic mechanisms of PD remain unclear, and no effective therapy currently exists to stop neurodegeneration in this debilitating disease. The identification of mutations in mitochondrial serine/threonine kinase PINK1 or E3 ubiquitin-protein ligase parkin as the cause of autosomal recessive PD opens up new avenues for uncovering neuroprotective pathways and PD pathogenic mechanisms. Recent studies reveal that PINK1 translocates to the outer mitochondrial membrane in response to mitochondrial depolarization and phosphorylates ubiquitin at the residue Ser65. The phosphorylated ubiquitin serves as a signal for activating parkin and recruiting autophagy receptors to promote clearance of damaged mitochondria via mitophagy. Emerging evidence has begun to indicate a link between impaired ubiquitin phosphorylation-dependent mitophagy and PD pathogenesis and supports the potential of Ser65-phosphorylated ubiquitin as a biomarker for PD. The new mechanistic insights and phenotypic screens have identified multiple potential therapeutic targets for PD drug discovery. This review highlights recent advances in understanding ubiquitin phosphorylation in mitochondrial quality control and PD pathogenesis and discusses how these findings can be translated into novel approaches for PD diagnostic and therapeutic development.
Keywords: Mitochondrial quality control; Mitophagy; PINK1; Parkin; Parkinson’s disease; Ubiquitin phosphorylation; Ubiquitin-protein ligase.
Figures

Similar articles
-
Binding to serine 65-phosphorylated ubiquitin primes Parkin for optimal PINK1-dependent phosphorylation and activation.EMBO Rep. 2015 Aug;16(8):939-54. doi: 10.15252/embr.201540352. Epub 2015 Jun 25. EMBO Rep. 2015. PMID: 26116755 Free PMC article.
-
Mitochondrial dysfunction in Parkinson's disease.Transl Neurodegener. 2016 Jul 22;5:14. doi: 10.1186/s40035-016-0060-6. eCollection 2016. Transl Neurodegener. 2016. PMID: 27453777 Free PMC article. Review.
-
Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65.Biochem J. 2014 May 15;460(1):127-39. doi: 10.1042/BJ20140334. Biochem J. 2014. PMID: 24660806 Free PMC article.
-
Dyrk1A phosphorylates parkin at Ser-131 and negatively regulates its ubiquitin E3 ligase activity.J Neurochem. 2015 Aug;134(4):756-68. doi: 10.1111/jnc.13164. Epub 2015 Jun 3. J Neurochem. 2015. PMID: 25963095
-
[Etiology and pathogenesis of Parkinson's disease: from mitochondrial dysfunctions to familial Parkinson's disease].Rinsho Shinkeigaku. 2004 Apr-May;44(4-5):241-62. Rinsho Shinkeigaku. 2004. PMID: 15287506 Review. Japanese.
Cited by
-
Protein modification in neurodegenerative diseases.MedComm (2020). 2024 Aug 4;5(8):e674. doi: 10.1002/mco2.674. eCollection 2024 Aug. MedComm (2020). 2024. PMID: 39105197 Free PMC article. Review.
-
Post-translational modification and mitochondrial function in Parkinson's disease.Front Mol Neurosci. 2024 Jan 11;16:1329554. doi: 10.3389/fnmol.2023.1329554. eCollection 2023. Front Mol Neurosci. 2024. PMID: 38273938 Free PMC article. Review.
-
Implications of ALS-Associated Mutations on Biochemical and Biophysical Features of hSOD1 and Aggregation Formation.Biochem Genet. 2024 Oct;62(5):3658-3680. doi: 10.1007/s10528-023-10619-y. Epub 2024 Jan 9. Biochem Genet. 2024. PMID: 38196030
-
Network Protein Interaction in Parkinson's Disease and Periodontitis Interplay: A Preliminary Bioinformatic Analysis.Genes (Basel). 2020 Nov 23;11(11):1385. doi: 10.3390/genes11111385. Genes (Basel). 2020. PMID: 33238395 Free PMC article.
-
Clustering of Alzheimer's and Parkinson's disease based on genetic burden of shared molecular mechanisms.Sci Rep. 2020 Nov 5;10(1):19097. doi: 10.1038/s41598-020-76200-4. Sci Rep. 2020. PMID: 33154531 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

