Size-controlled and redox-responsive supramolecular nanoparticles
- PMID: 26733345
- PMCID: PMC4685861
- DOI: 10.3762/bjoc.11.260
Size-controlled and redox-responsive supramolecular nanoparticles
Abstract
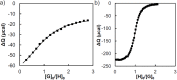
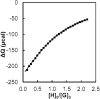
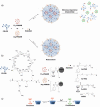
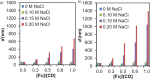
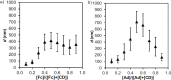
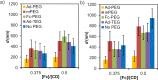
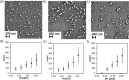
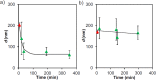
Control over the assembly and disassembly of nanoparticles is pivotal for their use as drug delivery vehicles. Here, we aim to form supramolecular nanoparticles (SNPs) by combining advantages of the reversible assembly properties of SNPs using host-guest interactions and of a stimulus-responsive moiety. The SNPs are composed of a core of positively charged poly(ethylene imine) grafted with β-cyclodextrin (CD) and a positively charged ferrocene (Fc)-terminated poly(amidoamine) dendrimer, with a monovalent stabilizer at the surface. Fc was chosen for its loss of CD-binding properties when oxidizing it to the ferrocenium cation. The ionic strength was shown to play an important role in controlling the aggregate growth. The attractive supramolecular and repulsive electrostatic interactions constitute a balance of forces in this system at low ionic strengths. At higher ionic strengths, the increased charge screening led to a loss of electrostatic repulsion and therefore to faster aggregate growth. A Job plot showed that a 1:1 stoichiometry of host and guest moieties gave the most efficient aggregate growth. Different stabilizers were used to find the optimal stopper to limit the growth. A weaker guest moiety was shown to be less efficient in stabilizing the SNPs. Also steric repulsion is important for achieving SNP stability. SNPs of controlled particle size and good stability (up to seven days) were prepared by fine-tuning the ratio of multivalent and monovalent interactions. Finally, reversibility of the SNPs was confirmed by oxidizing the Fc guest moieties in the core of the SNPs.
Keywords: host–guest interactions; nanoparticles; self-assembly; stimulus-responsive; supramolecular chemistry.
Figures

Similar articles
-
Cyclodextrin-based supramolecular nanoparticles stabilized by balancing attractive host-guest and repulsive electrostatic interactions.Chem Commun (Camb). 2014 Jul 14;50(55):7280-2. doi: 10.1039/c4cc03136a. Chem Commun (Camb). 2014. PMID: 24871809
-
Zwitterionic supramolecular nanoparticles: self-assembly and responsive properties.Nanoscale. 2015 May 7;7(17):7915-9. doi: 10.1039/c5nr01526j. Nanoscale. 2015. PMID: 25857281
-
Cyclodextrin-based host-guest supramolecular nanoparticles for delivery: from design to applications.Acc Chem Res. 2014 Jul 15;47(7):2017-25. doi: 10.1021/ar500055s. Epub 2014 May 29. Acc Chem Res. 2014. PMID: 24873201
-
Soft Supramolecular Nanoparticles by Noncovalent and Host-Guest Interactions.Small. 2016 Jan 6;12(1):96-119. doi: 10.1002/smll.201501348. Epub 2015 Nov 19. Small. 2016. PMID: 26584451 Review.
-
Emerging Supramolecular Therapeutic Carriers Based on Host-Guest Interactions.Chem Asian J. 2016 May 6;11(9):1300-21. doi: 10.1002/asia.201501434. Epub 2016 Feb 25. Chem Asian J. 2016. PMID: 26833861 Review.
Cited by
-
Nanotechnology as a Platform for the Development of Injectable Parenteral Formulations: A Comprehensive Review of the Know-Hows and State of the Art.Pharmaceutics. 2020 Jun 3;12(6):510. doi: 10.3390/pharmaceutics12060510. Pharmaceutics. 2020. PMID: 32503171 Free PMC article. Review.
-
Superstructures with cyclodextrins: chemistry and applications III.Beilstein J Org Chem. 2016 May 10;12:937-8. doi: 10.3762/bjoc.12.91. eCollection 2016. Beilstein J Org Chem. 2016. PMID: 27340483 Free PMC article. No abstract available.
-
Nanoparticles-mediated CRISPR-Cas9 gene therapy in inherited retinal diseases: applications, challenges, and emerging opportunities.J Nanobiotechnology. 2022 Dec 3;20(1):511. doi: 10.1186/s12951-022-01717-x. J Nanobiotechnology. 2022. PMID: 36463195 Free PMC article. Review.
References
-
- Chen K-J, Garcia M A, Wang H, Tseng H-R. Supramolecular Chemistry. John Wiley & Sons, Ltd; 2012. pp. 1–16.
LinkOut - more resources
Full Text Sources
Other Literature Sources
