Regeneration of Vocal Fold Mucosa Using Tissue-Engineered Structures with Oral Mucosal Cells
- PMID: 26730600
- PMCID: PMC4701435
- DOI: 10.1371/journal.pone.0146151
Regeneration of Vocal Fold Mucosa Using Tissue-Engineered Structures with Oral Mucosal Cells
Abstract
Objectives: Scarred vocal folds result in irregular vibrations during phonation due to stiffness of the vocal fold mucosa. To date, a completely satisfactory corrective procedure has yet to be achieved. We hypothesize that a potential treatment option for this disease is to replace scarred vocal folds with organotypic mucosa. The purpose of this study is to regenerate vocal fold mucosa using a tissue-engineered structure with autologous oral mucosal cells.
Study design: Animal experiment using eight beagles (including three controls).
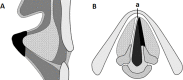
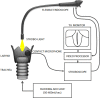
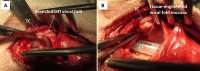
Methods: A 3 mm by 3 mm specimen of canine oral mucosa was surgically excised and divided into epithelial and subepithelial tissues. Epithelial cells and fibroblasts were isolated and cultured separately. The proliferated epithelial cells were co-cultured on oriented collagen gels containing the proliferated fibroblasts for an additional two weeks. The organotypic cultured tissues were transplanted to the mucosa-deficient vocal folds. Two months after transplantation, vocal fold vibrations and morphological characteristics were observed.
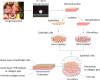
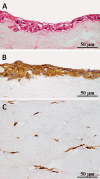
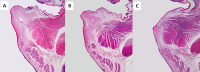
Results: A tissue-engineered vocal fold mucosa, consisting of stratified epithelium and lamina propria, was successfully fabricated to closely resemble the normal layered vocal fold mucosa. Laryngeal stroboscopy revealed regular but slightly small mucosal waves at the transplanted site. Immunohistochemically, stratified epithelium expressed cytokeratin, and the distributed cells in the lamina propria expressed vimentin. Elastic Van Gieson staining revealed a decreased number of elastic fibers in the lamina propria of the transplanted site.
Conclusion: The fabricated mucosa with autologous oral mucosal cells successfully restored the vocal fold mucosa. This reconstruction technique could offer substantial clinical advantages for treating intractable diseases such as scarring of the vocal folds.
Conflict of interest statement
Figures




Similar articles
-
Lamina propria replacement therapy with cultured autologous fibroblasts for vocal fold scars.Otolaryngol Head Neck Surg. 2004 Dec;131(6):864-70. doi: 10.1016/j.otohns.2004.07.010. Otolaryngol Head Neck Surg. 2004. PMID: 15577782
-
Tissue-Engineered Vocal Fold Mucosa Implantation in Rabbits.Otolaryngol Head Neck Surg. 2016 Apr;154(4):679-88. doi: 10.1177/0194599816628501. Epub 2016 Mar 8. Otolaryngol Head Neck Surg. 2016. PMID: 26956198 Free PMC article.
-
[Characterization of vocal fold regeneration after adipose-derived mesenchymal stem cells implanting].Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010 Sep;45(9):723-8. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010. PMID: 21092668 Chinese.
-
Tissue engineering-based therapeutic strategies for vocal fold repair and regeneration.Biomaterials. 2016 Nov;108:91-110. doi: 10.1016/j.biomaterials.2016.08.054. Epub 2016 Sep 2. Biomaterials. 2016. PMID: 27619243 Free PMC article. Review.
-
Current treatment of vocal fold scarring.Curr Opin Otolaryngol Head Neck Surg. 2005 Jun;13(3):143-7. doi: 10.1097/01.moo.0000162261.49739.b7. Curr Opin Otolaryngol Head Neck Surg. 2005. PMID: 15908810 Review.
Cited by
-
Comparative proteomics of paired vocal fold and oral mucosa fibroblasts.J Proteomics. 2017 Feb 23;155:11-21. doi: 10.1016/j.jprot.2017.01.010. Epub 2017 Jan 15. J Proteomics. 2017. PMID: 28099887 Free PMC article.
-
Motor endplate-expressing cartilage-muscle implants for reconstruction of a denervated hemilarynx.Laryngoscope. 2019 Jun;129(6):1293-1300. doi: 10.1002/lary.27575. Epub 2018 Dec 12. Laryngoscope. 2019. PMID: 30548608 Free PMC article.
-
Recent trends and perspectives in reconstruction and regeneration of intra/extra-oral wounds using tissue-engineered oral mucosa equivalents.Jpn Dent Sci Rev. 2023 Dec;59:365-374. doi: 10.1016/j.jdsr.2023.10.002. Epub 2023 Oct 25. Jpn Dent Sci Rev. 2023. PMID: 37954029 Free PMC article. Review.
-
Optimized Generation of Primary Human Epithelial Cells from Larynx and Hypopharynx: A Site-Specific Epithelial Model for Reflux Research.Cell Transplant. 2019 May;28(5):630-637. doi: 10.1177/0963689719838478. Epub 2019 Mar 27. Cell Transplant. 2019. PMID: 30917697 Free PMC article.
-
Introducing a new type of alternative laryngeal mucosa model.PLoS One. 2023 Jun 30;18(6):e0287634. doi: 10.1371/journal.pone.0287634. eCollection 2023. PLoS One. 2023. PMID: 37390090 Free PMC article.
References
-
- Hirano M. Morphological structure of the vocal cord as a vibrator and its variations. Folia Phoniatr (Basel). 1974;26: 89–94. - PubMed
-
- Kurita S, Nagata K, Hirano M. A comparative study of the layer structure of the vocal fold College-Hill: Vocal fold physiology San Diego; 1983:3–21.
-
- Isshiki N, shoji K, Kojima H, Hirano S. Vocal fold atrophy and its surgical treatment. Ann Otol Rhinol Laryngol. 1996;105: 182–188. - PubMed
-
- Isshiki N. Progress in laryngeal framework surgery. Acta Otolaryngol. 2000;120: 120–127. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

